Motion-robust diffusion compartment imaging using simultaneous multi-slice acquisition
- PMID: 30443929
- PMCID: PMC6414287
- DOI: 10.1002/mrm.27613
Motion-robust diffusion compartment imaging using simultaneous multi-slice acquisition
Abstract
Purpose: To achieve motion-robust diffusion compartment imaging (DCI) in near continuously moving subjects based on simultaneous multi-slice, diffusion-weighted brain MRI.
Methods: Simultaneous multi-slice (SMS) acquisition enables fast and dense sampling of k- and q-space. We propose to achieve motion-robust DCI via slice-level motion correction by exploiting the rigid coupling between simultaneously acquired slices. This coupling provides 3D coverage of the anatomy that substantially constraints the slice-to-volume alignment problem. This is incorporated into an explicit model of motion dynamics that handles continuous and large subject motion in robust DCI reconstruction.
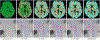
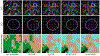
Results: We applied the proposed technique, called Motion Tracking based on Simultanous Multislice Registration (MT-SMR) to multi b-value SMS diffusion-weighted brain MRI of healthy volunteers and motion-corrupted scans of 20 pediatric subjects. Quantitative and qualitative evaluation based on fractional anisotropy in unidirectional fiber regions, and DCI in crossing-fiber regions show robust reconstruction in the presence of motion.
Conclusion: The proposed approach has the potential to extend routine use of SMS DCI in very challenging populations, such as young children, newborns, and non-cooperative patients.
Keywords: Diffusion-compartment imaging; diffusion-weighted MRI; image-based navigation; intra-volume motion; motion tracking; motion-robust; simultaneous multi-slice; slice registration.
© 2018 International Society for Magnetic Resonance in Medicine.
Figures


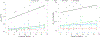



References
-
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M, MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders., Radiology 161 (2) (1986) 401–407. - PubMed
-
- Le Bihan D, Turner R, Douek P, Patronas N, Diffusion MR imaging: clinical applications., AJR. American journal of roentgenology 159 (3) (1992) 591–599. - PubMed
-
- Basser PJ, Jones DK, Diffusion-tensor MRI: theory, experimental design and data analysis-a technical review, NMR in Biomedicine 15 (7–8) (2002) 456–467. - PubMed
-
- Basser PJ, Pierpaoli C, Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI, Journal of magnetic resonance 213 (2) (2011) 560–570. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

