Toward predictive modeling of catheter-based pulmonary valve replacement into native right ventricular outflow tracts
- PMID: 30444053
- PMCID: PMC7713792
- DOI: 10.1002/ccd.27962
Toward predictive modeling of catheter-based pulmonary valve replacement into native right ventricular outflow tracts
Abstract
Background: Pulmonary insufficiency is a consequence of transannular patch repair in Tetralogy of Fallot (ToF) leading to late morbidity and mortality. Transcatheter native outflow tract pulmonary valve replacement has become a reality. However, predicting a secure, atraumatic implantation of a catheter-based device remains a significant challenge due to the complex and dynamic nature of the right ventricular outflow tract (RVOT). We sought to quantify the differences in compression and volume for actual implants, and those predicted by pre-implant modeling.
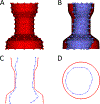
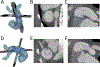
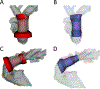
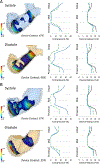
Methods: We used custom software to interactively place virtual transcatheter pulmonary valves (TPVs) into RVOT models created from pre-implant and post Harmony valve implant CT scans of 5 ovine surgical models of TOF to quantify and visualize device volume and compression.
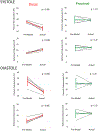
Results: Virtual device placement visually mimicked actual device placement and allowed for quantification of device volume and radius. On average, simulated proximal and distal device volumes and compression did not vary statistically throughout the cardiac cycle (P = 0.11) but assessment was limited by small sample size. In comparison to actual implants, there was no significant pairwise difference in the proximal third of the device (P > 0.80), but the simulated distal device volume was significantly underestimated relative to actual device implant volume (P = 0.06).
Conclusions: This study demonstrates that pre-implant modeling which assumes a rigid vessel wall may not accurately predict the degree of distal RVOT expansion following actual device placement. We suggest the potential for virtual modeling of TPVR to be a useful adjunct to procedural planning, but further development is needed.
Keywords: magnetic resonance imaging; percutaneous pulmonary valve implantation; prosthetic heart valve; tetralogy of Fallot.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
DISCLOSURES
Drs. Gillespie and Morray serve as consultants for Medtronic, the manufacturer of the Harmony Device. The other authors report no conflicts.
Figures



References
-
- Al Habib HF, Jacobs JP, Mavroudis C, Tchervenkov CI, O’Brien SM, Mohammadi S, Jacobs ML. Contemporary patterns of management of tetralogy of Fallot: data from the Society of Thoracic Surgeons Database. Ann Thorac Surg 2010;90(3):813–9; discussion 819–20. - PubMed
-
- Apitz C, Webb GD, Redington AN. Tetralogy of fallot. The Lancet 2009;374(9699):1462–1471. - PubMed
-
- Schoonbeek RC, Takebayashi S, Aoki C, Shimaoka T, Harris MA, Fu GL, Kim TS, Dori Y, McGarvey J, Litt H and others. Implantation of the Medtronic Harmony Transcatheter Pulmonary Valve Improves Right Ventricular Size and Function in an Ovine Model of Postoperative Chronic Pulmonary Insufficiency. Circ Cardiovasc Interv 2016;9(10). - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

