Depth dependence of coherent hemodynamics in the human head
- PMID: 30444084
- PMCID: PMC6318717
- DOI: 10.1117/1.JBO.23.12.121615
Depth dependence of coherent hemodynamics in the human head
Abstract
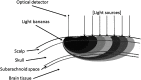
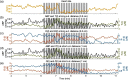
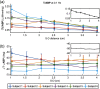
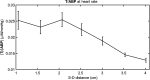
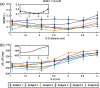
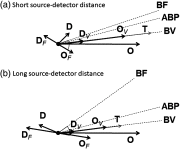
We report a near-infrared spectroscopy (NIRS) study of coherent hemodynamic oscillations measured on the human forehead at multiple source-detector distances (1 to 4 cm). The physiological source of the coherent hemodynamics is arterial blood pressure oscillations at a frequency of 0.1 Hz, induced by cyclic inflation (to a pressure of 200 mmHg) and deflation of two thigh cuffs wrapped around the subject's thighs. To interpret our results, we use a recently developed hemodynamic model and a phasor representation of the oscillations of oxyhemoglobin, deoxyhemoglobin, and total hemoglobin concentrations in the tissue (phasors O, D, and T, respectively). The increase in the phase angle between D and O at larger source-detector separations is assigned to greater flow versus volume contributions and to a stronger blood flow autoregulation in deeper tissue (brain cortex) with respect to superficial tissue (scalp and skull). The relatively constant phase lag of T versus arterial blood pressure oscillations at all source-detector distances was assigned to competing effects from stronger autoregulation and smaller arterial-to-venous contributions in deeper tissue with respect to superficial tissue. We demonstrate the application of a hemodynamic model to interpret coherent hemodynamics measured with NIRS and to assess the different nature of shallow (extracerebral) versus deep (cerebral) tissue hemodynamics.
Keywords: brain perfusion; cerebral autoregulation; coherent hemodynamics; hemoglobin concentration; near-infrared spectroscopy; phasors.
(2018) COPYRIGHT Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures


References
-
- Fantini S., “Dynamic model for the tissue concentration and oxygen saturation of hemoglobin in relation to blood volume, flow velocity, and oxygen consumption: implications for functional neuroimaging and coherent hemodynamics spectroscopy (CHS),” NeuroImage 85(1), 202–221 (2014).NEIMEF10.1016/j.neuroimage.2013.03.065 - DOI - PMC - PubMed

