Quantitative determination of a model organic/insulator/metal interface structure
- PMID: 30444513
- PMCID: PMC6289171
- DOI: 10.1039/c8nr06387g
Quantitative determination of a model organic/insulator/metal interface structure
Abstract
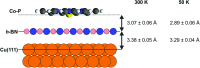
By combining X-ray photoelectron spectroscopy, X-ray standing waves and scanning tunneling microscopy, we investigate the geometric and electronic structure of a prototypical organic/insulator/metal interface, namely cobalt porphine on monolayer hexagonal boron nitride (h-BN) on Cu(111). Specifically, we determine the adsorption height of the organic molecule and show that the original planar molecular conformation is preserved in contrast to the adsorption on Cu(111). In addition, we highlight the electronic decoupling provided by the h-BN spacer layer and find that the h-BN-metal separation is not significantly modified by the molecular adsorption. Finally, we find indication of a temperature dependence of the adsorption height, which might be a signature of strongly-anisotropic thermal vibrations of the weakly bonded molecules.
Figures


Similar articles
-
Hybridization vs decoupling: influence of an h-BN interlayer on the physical properties of a lander-type molecule on Ni(111).Beilstein J Nanotechnol. 2020 Aug 4;11:1168-1177. doi: 10.3762/bjnano.11.101. eCollection 2020. Beilstein J Nanotechnol. 2020. PMID: 32821641 Free PMC article.
-
Corrugation in the Weakly Interacting Hexagonal-BN/Cu(111) System: Structure Determination by Combining Noncontact Atomic Force Microscopy and X-ray Standing Waves.ACS Nano. 2017 Sep 26;11(9):9151-9161. doi: 10.1021/acsnano.7b04022. Epub 2017 Sep 5. ACS Nano. 2017. PMID: 28872822
-
Interplay between Energy-Level Position and Charging Effect of Manganese Phthalocyanines on an Atomically Thin Insulator.ACS Nano. 2015 Oct 27;9(10):10125-32. doi: 10.1021/acsnano.5b03741. Epub 2015 Sep 25. ACS Nano. 2015. PMID: 26390030
-
Layered Insulator/Molecule/Metal Heterostructures with Molecular Functionality through Porphyrin Intercalation.ACS Nano. 2018 Mar 27;12(3):2677-2684. doi: 10.1021/acsnano.7b08887. Epub 2018 Mar 2. ACS Nano. 2018. PMID: 29498827
-
Templated self-assembly and local doping of molecules on epitaxial hexagonal boron nitride.ACS Nano. 2013 Dec 23;7(12):11121-8. doi: 10.1021/nn404840h. Epub 2013 Oct 25. ACS Nano. 2013. PMID: 24152095
Cited by
-
Self-assembly of C60 on a ZnTPP/Fe(001)-p(1 × 1)O substrate: observation of a quasi-freestanding C60 monolayer.Beilstein J Nanotechnol. 2022 Aug 30;13:857-864. doi: 10.3762/bjnano.13.76. eCollection 2022. Beilstein J Nanotechnol. 2022. PMID: 36105692 Free PMC article.
-
Hybridization vs decoupling: influence of an h-BN interlayer on the physical properties of a lander-type molecule on Ni(111).Beilstein J Nanotechnol. 2020 Aug 4;11:1168-1177. doi: 10.3762/bjnano.11.101. eCollection 2020. Beilstein J Nanotechnol. 2020. PMID: 32821641 Free PMC article.
-
Self-assembly and spectroscopic fingerprints of photoactive pyrenyl tectons on hBN/Cu(111).Beilstein J Nanotechnol. 2020 Sep 29;11:1470-1483. doi: 10.3762/bjnano.11.130. eCollection 2020. Beilstein J Nanotechnol. 2020. PMID: 33083195 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

