CLARITY reveals a more protracted temporal course of axon swelling and disconnection than previously described following traumatic brain injury
- PMID: 30444552
- PMCID: PMC6482960
- DOI: 10.1111/bpa.12677
CLARITY reveals a more protracted temporal course of axon swelling and disconnection than previously described following traumatic brain injury
Abstract
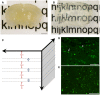
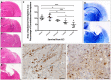
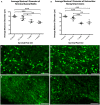
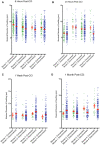
Diffuse axonal injury (DAI) is an important consequence of traumatic brain injury (TBI). At the moment of trauma, axons rarely disconnect, but undergo cytoskeletal disruption and transport interruption leading to protein accumulation within swellings. The amyloid precursor protein (APP) accumulates rapidly and the standard histological evaluation of axonal pathology relies upon its detection. APP+ swellings first appear as varicosities along intact axons, which can ultimately undergo secondary disconnection to leave a terminal "axon bulb" at the disconnected, proximal end. However, sites of disconnection are difficult to determine with certainty using standard, thin tissue sections, thus limiting the comprehensive evaluation of axon degeneration. The tissue-clearing technique, CLARITY, permits three-dimensional visualization of axons that would otherwise be out of plane in standard tissue sections. Here, we examined the morphology and connection status of APP+ swellings using CLARITY at 6 h, 24 h, 1 week and 1 month following the controlled cortical impact (CCI) model of TBI in mice. Remarkably, many APP+ swellings that appeared as terminal bulbs when viewed in standard 8-µm-thick regions of tissue were instead revealed to be varicose swellings along intact axons when three dimensions were fully visible. Moreover, the percentage of these potentially viable axon swellings differed with survival from injury and may represent the delayed onset of distinct mechanisms of degeneration. Even at 1-month post-CCI, ~10% of apparently terminal bulbs were revealed as connected by CLARITY and are thus potentially salvageable. Intriguingly, the diameter of swellings decreased with survival, including varicosities along intact axons, and may reflect reversal of, or reduced, axonal transport interruption in the chronic setting. These data indicate that APP immunohistochemistry on standard thickness tissue sections overestimates axon disconnection, particularly acutely post-injury. Evaluating cleared tissue demonstrates a surprisingly delayed process of axon disconnection and thus longer window of therapeutic opportunity than previously appreciated. Intriguingly, a subset of axon swellings may also be capable of recovery.
Keywords: CLARITY; TBI; amyloid precursor protein; axon degeneration; axonal pathology; diffuse axonal injury; traumatic brain injury.
© 2018 International Society of Neuropathology.
Conflict of interest statement
No authors have any conflicts of interest.
Figures

References
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR (1989) Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 15:49–59. - PubMed
-
- Adams JH, Doyle D, Graham DI, Lawrence AE, Mclellan DR (1984) Diffuse axonal injury in head injuries caused by a fall. Lancet 2:1420–1422. - PubMed
-
- Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 12:557–563. - PubMed
-
- Anderson CV, Bigler ED (1995) Ventricular dilation, cortical atrophy, and neuropsychological outcome following traumatic brain injury. J Neuropsychiatry Clin Neurosci 7:42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

