Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence
- PMID: 30444745
- PMCID: PMC6416096
- DOI: 10.1097/MEG.0000000000001311
Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence
Abstract
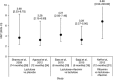
A consolidated overview of evidence for the effectiveness and safety/tolerability of hepatic encephalopathy (HE) treatment over the long term is currently lacking. We identified and assessed published evidence for the long-term (≥6 months) pharmacological management of HE with lactulose and/or rifaximin. A literature search was conducted in PubMed (cutoff date 05 March 2018) using the search terms 'hepatic encephalopathy+rifaximin' and 'hepatic encephalopathy+lactulose'. All articles containing primary clinical data were manually assessed to identify studies in which long-term (≥6 months) effectiveness and/or safety/tolerability end points were reported for lactulose and/or rifaximin. Long-term effectiveness outcomes were reported in eight articles for treatment with lactulose alone and 19 articles for treatment with rifaximin, alone or in combination with lactulose. Long-term safety/tolerability outcomes were reported in six articles for treatment with lactulose alone and nine articles for treatment with rifaximin, alone or in combination with lactulose. These studies showed that lactulose is effective for the prevention of overt HE recurrence over the long term and that the addition of rifaximin to lactulose significantly reduces the risk of overt HE recurrence and HE-related hospitalization, compared with lactulose therapy alone, without compromising tolerability. Current evidence therefore supports recommendations for the use of lactulose therapy for the prevention of overt HE recurrence over the long term, and for the additional benefit of adding rifaximin to lactulose therapy. Addition of rifaximin to standard lactulose therapy may result in substantial reductions in healthcare resource utilization over the long term, by reducing overt HE recurrence and associated rehospitalization.
Figures

References
-
- American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014; 61:642–659. - PubMed
-
- Williams R. Review article: bacterial flora and pathogenesis in hepatic encephalopathy. Aliment Pharmacol Ther 2007; 25 (Suppl 1):17–22. - PubMed
-
- Cordoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, et al. CANONIC Study Investigators of EASL-CLIF Consortium. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014; 60:275–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

