Experimental co-transmission of Simian Immunodeficiency Virus (SIV) and the macaque homologs of the Kaposi Sarcoma-Associated Herpesvirus (KSHV) and Epstein-Barr Virus (EBV)
- PMID: 30444879
- PMCID: PMC6239284
- DOI: 10.1371/journal.pone.0205632
Experimental co-transmission of Simian Immunodeficiency Virus (SIV) and the macaque homologs of the Kaposi Sarcoma-Associated Herpesvirus (KSHV) and Epstein-Barr Virus (EBV)
Abstract
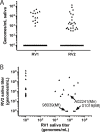
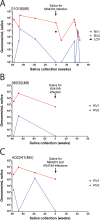
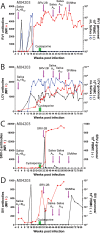
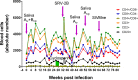
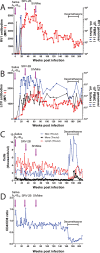
Macaque RFHV and LCV are close homologs of human KSHV and EBV, respectively. No experimental model of RFHV has been developed due to the lack of a source of culturable infectious virus. Screening of macaques at the Washington National Primate Research Center detected RFHV in saliva of SIV-infected macaques from previous vaccine studies. A pilot experimental infection of two naïve juvenile pig-tailed macaques was initiated by inoculation of saliva from SIV-infected pig-tailed and cynomolgus macaque donors, which contained high levels of DNA (> 10(6) genomes/ml) of the respective species-specific RFHV strain. Both juvenile recipients developed SIV and RFHV infections with RFHV DNA detected transiently in saliva and/or PBMC around week 16 post-infection. One juvenile macaque was infected with the homologous RFHVMn from whole saliva of a pig-tailed donor, which had been inoculated into the cheek pouch. This animal became immunosuppressed, developing simian AIDS and was euthanized 23 weeks after inoculation. The levels of RFHV DNA in saliva and PBMC remained below the level of detection after week 17, showing no reactivation of the RFHVMn infection during the rapid development of AIDS. The other juvenile macaque was infected with the heterologous RFHVMf from i.v. inoculation of purified virions from saliva of a cynomolgus donor. The juvenile recipient remained immunocompetent, developing high levels of persistent anti-RFHV and -SIV antibodies. After the initial presence of RFHVMf DNA in saliva and PBMC decreased to undetectable levels by week 19, all attempts to reactivate the infection through additional inoculations, experimental infection with purified SRV-2 or SIV, or immunosuppressive treatments with cyclosporine or dexamethasone were unsuccessful. An heterologous LCV transmission was also detected in this recipient, characterized by continual high levels of LCVMf DNA from the cynomolgus donor in both saliva (> 10(6) genomes/ml) and PBMC (> 10(4) genomes/million cells), coupled with high levels of anti-LCV antibodies. The macaque was sacrificed 209 weeks after the initial inoculation. Low levels of LCVMf DNA were detected in salivary glands, tonsils and other lymphoid organs, while RFHVMf DNA was below the level of detection. These results show successful co-transmission of RFHV and LCV from saliva and demonstrate differential lytic activation of the different gammaherpesvirus lineages due to presumed differences in biology and tropism and control by the host immune system. Although this initial pilot transmission study utilized only two macaques, it provides the first evidence for experimental transmission of the macaque homolog of KSHV, setting the stage for larger transmission studies to examine the differential activation of rhadinovirus and lymphocryptovirus infections and the pathological effects of immunosuppression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Macaque homologs of EBV and KSHV show uniquely different associations with simian AIDS-related lymphomas.PLoS Pathog. 2012;8(10):e1002962. doi: 10.1371/journal.ppat.1002962. Epub 2012 Oct 4. PLoS Pathog. 2012. PMID: 23055934 Free PMC article.
-
Comparative Analysis of Gammaherpesvirus Circular RNA Repertoires: Conserved and Unique Viral Circular RNAs.J Virol. 2019 Mar 5;93(6):e01952-18. doi: 10.1128/JVI.01952-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30567979 Free PMC article.
-
Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses.J Virol. 1999 Dec;73(12):10320-8. doi: 10.1128/JVI.73.12.10320-10328.1999. J Virol. 1999. PMID: 10559350 Free PMC article.
-
Non-human Primate Lymphocryptoviruses: Past, Present, and Future.Curr Top Microbiol Immunol. 2015;391:385-405. doi: 10.1007/978-3-319-22834-1_13. Curr Top Microbiol Immunol. 2015. PMID: 26428382 Review.
-
Rhesus monkey rhadinovirus: a model for the study of KSHV.Curr Top Microbiol Immunol. 2007;312:43-69. doi: 10.1007/978-3-540-34344-8_2. Curr Top Microbiol Immunol. 2007. PMID: 17089793 Review.
Cited by
-
Probing Reconstituted Human Immune Systems in Mice With Oncogenic γ-Herpesvirus Infections.Front Immunol. 2020 Sep 9;11:581419. doi: 10.3389/fimmu.2020.581419. eCollection 2020. Front Immunol. 2020. PMID: 33013936 Free PMC article. Review.
-
Dynamic alterations in bacterial and fungal microbiome and inflammatory cytokines following SRV-8 infection in cynomolgus monkeys.Zool Res. 2025 Mar 18;46(2):325-338. doi: 10.24272/j.issn.2095-8137.2024.278. Zool Res. 2025. PMID: 40049661 Free PMC article.
-
Modulation of Epstein-Barr-Virus (EBV)-Associated Cancers by Co-Infections.Cancers (Basel). 2023 Dec 7;15(24):5739. doi: 10.3390/cancers15245739. Cancers (Basel). 2023. PMID: 38136285 Free PMC article. Review.
-
Roles of Lytic Viral Replication and Co-Infections in the Oncogenesis and Immune Control of the Epstein-Barr Virus.Cancers (Basel). 2021 May 10;13(9):2275. doi: 10.3390/cancers13092275. Cancers (Basel). 2021. PMID: 34068598 Free PMC article. Review.
-
The Role of Lytic Infection for Lymphomagenesis of Human γ-Herpesviruses.Front Cell Infect Microbiol. 2021 Mar 26;11:605258. doi: 10.3389/fcimb.2021.605258. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33842383 Free PMC article. Review.
References
-
- Mariggio G, Koch S, Schulz TF. Kaposi sarcoma herpesvirus pathogenesis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2017;372(1732). 10.1098/rstb.2016.0275 ; PubMed Central PMCID: PMC5597742. - DOI - PMC - PubMed
-
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nature reviews Cancer. 2004;4(10):757–68. 10.1038/nrc1452 . - DOI - PubMed
-
- Krithivas A, Young DB, Liao G, Greene D, Hayward SD. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J Virol. 2000;74(20):9637–45. ; PubMed Central PMCID: PMC112396. - PMC - PubMed
-
- Xu D, Coleman T, Zhang J, Fagot A, Kotalik C, Zhao L, et al. Epstein-Barr virus inhibits Kaposi's sarcoma-associated herpesvirus lytic replication in primary effusion lymphomas. J Virol. 2007;81(11):6068–78. 10.1128/JVI.02743-06 ; PubMed Central PMCID: PMC1900272. - DOI - PMC - PubMed
-
- Jiang Y, Xu D, Zhao Y, Zhang L. Mutual inhibition between Kaposi's sarcoma-associated herpesvirus and Epstein-Barr virus lytic replication initiators in dually-infected primary effusion lymphoma. PloS one. 2008;3(2):e1569 10.1371/journal.pone.0001569 ; PubMed Central PMCID: PMC2215330. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

