Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase
- PMID: 30444932
- PMCID: PMC6508893
- DOI: 10.1002/chem.201805075
Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase
Abstract
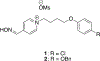
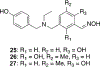
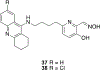
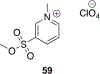
Organophosphorus (OP) nerve agents and pesticides present significant threats to civilian and military populations. OP compounds include the nefarious G and V chemical nerve agents, but more commonly, civilians are exposed to less toxic OP pesticides, resulting in the same negative toxicological effects and thousands of deaths on an annual basis. After decades of research, no new therapeutics have been realized since the mid-1900s. Upon phosphylation of the catalytic serine residue, a process known as inhibition, there is an accumulation of acetylcholine (ACh) in the brain synapses and neuromuscular junctions, leading to a cholinergic crisis and eventually death. Oxime nucleophiles can reactivate select OP-inhibited acetylcholinesterase (AChE). Yet, the fields of reactivation of AChE and butyrylcholinesterase encounter additional challenges as broad-spectrum reactivation of either enzyme is difficult. Additional problems include the ability to cross the blood brain barrier (BBB) and to provide therapy in the central nervous system. Yet another complication arises in a competitive reaction, known as aging, whereby OP-inhibited AChE is converted to an inactive form, which until very recently, had been impossible to reverse to an active, functional form. Evaluations of uncharged oximes and other neutral nucleophiles have been made. Non-oxime reactivators, such as aromatic general bases and Mannich bases, have been developed. The issue of aging, which generates an anionic phosphylated serine residue, has been historically recalcitrant to recovery by any therapeutic approach-that is, until earlier this year. Mannich bases not only serve as reactivators of OP-inhibited AChE, but this class of compounds can also recover activity from the aged form of AChE, a process referred to as resurrection. This review covers the modern efforts to address all of these issues and notes the complexities of therapeutic development along these different lines of research.
Keywords: acetylcholinesterase; butyrylcholinesterase; organophosphorus; reactivation; resurrection.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
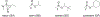
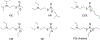
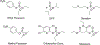
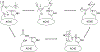
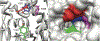
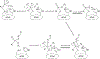


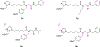
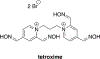

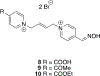
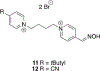
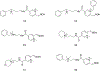

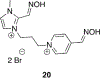

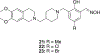
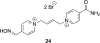

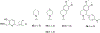
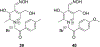

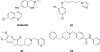
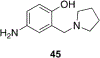
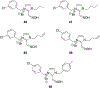
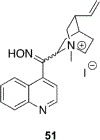
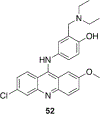
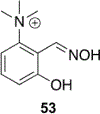

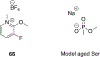
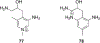
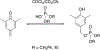
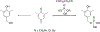
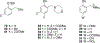
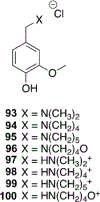
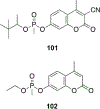
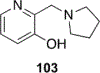
References
-
- Everts S, Chem. Eng. News 2016, 94, 26–28.
-
- Christianson S Fatal Airs : The Deadly History and Apocalyptic Future of Lethal Gases That Threaten Our World, Praeger, Santa Barbara, 2010, pp. 73–76.
-
- Gillispie CC, Holmes FL, Koertge N, Complete Dictionary of Scientific Biography, Thomson Gale, Detroit, 2008.
-
- Pruitt S, https://www.history.com/news/the-nazis-developed-sarin-gasbut-hitler-was..., 2017, 18–21.
-
- Minelle B, https://news.sky.com/story/vx-nerve-agent-what-is-it-wheredid-it-come-fr..., Sky News 2017, 1–12.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

