Chemical and Physiological Features of Mitochondrial Acylation
- PMID: 30444998
- PMCID: PMC6498146
- DOI: 10.1016/j.molcel.2018.10.023
Chemical and Physiological Features of Mitochondrial Acylation
Abstract
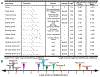
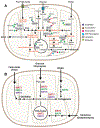
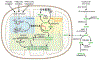
Growing appreciation of the diversity of post-translational modifications (PTMs) in the mitochondria necessitates reevaluation of the roles these modifications play in both health and disease. Compared to the cytosol and nucleus, the mitochondrial proteome is highly acylated, and remodeling of the mitochondrial "acylome" is a key adaptive mechanism that regulates fundamental aspects of mitochondrial biology. It is clear that we need to understand the underlying chemistry that regulates mitochondrial acylation, as well as how chemical properties of the acyl chain impact biological functions. Here, we dissect the sources of PTMs in the mitochondria, review major mitochondrial pathways that control levels of PTMs, and highlight how sirtuin enzymes respond to the bioenergetic state of the cell via NAD+ availability to regulate mitochondrial biology. By providing a framework connecting the chemistry of these modifications, their biochemical consequences, and the pathways that regulate the levels of acyl PTMs, we will gain a deeper understanding of the physiological significance of mitochondrial acylation and its role in mitochondrial adaptation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
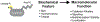
References
-
- Aksoy P, White TA, Thompson M, and Chini EN (2006). Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun 345, 1386–1392. - PubMed
-
- Alano CC, Tran A, Tao R, Ying W, Karliner JS, and Swanson RA (2007). Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J. Neurosci. Res 85, 3378–3385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

