In Vitro and In Silico Analyses of Nicotine Release from a Gelisphere-Loaded Compressed Polymeric Matrix for Potential Parkinson's Disease Interventions
- PMID: 30445765
- PMCID: PMC6320845
- DOI: 10.3390/pharmaceutics10040233
In Vitro and In Silico Analyses of Nicotine Release from a Gelisphere-Loaded Compressed Polymeric Matrix for Potential Parkinson's Disease Interventions
Abstract
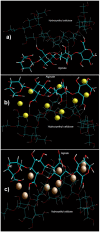
This study aimed to develop a prolonged-release device for the potential site-specific delivery of a neuroprotective agent (nicotine). The device was formulated as a novel reinforced crosslinked composite polymeric system with the potential for intrastriatal implantation in Parkinson's disease interventions. Polymers with biocompatible and bioerodible characteristics were selected to incorporate nicotine within electrolyte-crosslinked alginate-hydroxyethylcellulose gelispheres compressed within a release rate-modulating external polymeric matrix, comprising either hydroxypropylmethylcellulose (HPMC), polyethylene oxide (PEO), or poly(lactic-co-glycolic) acid (PLGA) to prolong nicotine release. The degradation and erosion studies showed that the produced device had desirable robustness with the essential attributes for entrapping drug molecules and retarding their release. Zero-order drug release was observed over 50 days from the device comprising PLGA as the external matrix. Furthermore, the alginate-nicotine interaction, the effects of crosslinking on the alginate-hydroxyethycellulose (HEC) blend, and the effects of blending PLGA, HPMC, and PEO on device performance were mechanistically elucidated using molecular modelling simulations of the 3D structure of the respective molecular complexes to predict the molecular interactions and possible geometrical orientation of the polymer morphologies affecting the geometrical preferences. The compressed polymeric matrices successfully retarded the release of nicotine over several days. PLGA matrices offered minimal rates of matrix degradation and successfully retarded nicotine release, leading to the achieved zero-order release for 50 days following exposure to simulated cerebrospinal fluid (CSF).
Keywords: PLGA discs; alginate gelispheres; crosslinked matrices; powder flow properties; prolonged release; textural analysis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Mouhape C., Costa G., Ferreira M., Abin-Carriquiry J.A., Dajas F., Prunell G. Nicotine-induced neuroprotection in rotenone in vivo and in vitro models of Parkinson’s disease: Evidences for the involvement of the labile iron pool level as the underlying mechanism. Neurotox. Res. 2018 doi: 10.1007/s12640-018-9931-1. in press. - DOI - PubMed
-
- Villafane G., Thiriez C., Audureau E., Straczek C., Kerschen P., Cormier-Dequaire F., Van Der Gucht A., Gurruchaga J.-M., Quéré-Carne M., Paul M., et al. High-dose transdermal nicotine in Parkinson’s disease patients: A randomized, open-label, blinded-endpoint evaluation phase 2 study. Eur. J. Neurol. 2018;25:120–127. doi: 10.1111/ene.13474. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

