The efficacy of paritaprevir/ritonavir/ombitasvir+dasabuvir and ledipasvir/sofosbuvir is comparable in patients who failed interferon-based treatment with first generation protease inhibitors - a multicenter cohort study
- PMID: 30445916
- PMCID: PMC6240185
- DOI: 10.1186/s12879-018-3465-2
The efficacy of paritaprevir/ritonavir/ombitasvir+dasabuvir and ledipasvir/sofosbuvir is comparable in patients who failed interferon-based treatment with first generation protease inhibitors - a multicenter cohort study
Abstract
Background: According to the EASL and AASLD guidelines, the recommended treatment for patients who failed to achieve a sustained virologic response (SVR) on prior interferon-based triple therapy with protease inhibitors (PI), is a combination of sofosbuvir and NS5A inhibitors. Polish national recommendations also allow the use of paritaprevir/ritonavir/ombitasvir+dasasbuvir±ribavirin (PrODR) in this group of patients. The aim of the study was to evaluate the efficacy and safety of PrODR vs. ledipasvir/sofosbuvir±RBV (LSR) in PI-experienced patients in real-life setting.
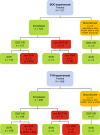
Methods: Our analysis included patients registered in the nationwide, investigators initiated, multicentre EpiTer-2 database. Among 4530 patients registered, 335 with genotype 1 (93% 1b) were previously treated with IFN-based regimens with PIs: 127 with boceprevir (BOC), 208 with telaprevir (TVR). Patients with advanced fibrosis (F3/F4) were significantly predominant (BOC 28.4%/61.4%, TVR 18.8%/64.4%, respectively). Subjects were assigned to IFN-free retreatment as follows: BOC - 64 (50.4%) PrODR and 63 (49.6%) LSR; TVR- 103 (49.5%) PrODR and 105 (50.5%) LSR.
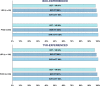
Results: SVR rates were comparable for particular groups: BOC → PrODR- 100%; BOC → LSR - 98%; TVR → PrODR - 97%; TVR → LSR - 96% (intent-to treat analysis-ITT) and BOC → PrODR→100%; BOC → LSR - 99%; TVR → PrODR - 99%; TVR → LSR - 98% (modified intent-to treat analysis-mITT). Both treatment regimens had a favourable safety profile. Adverse events (AEs) were generally mild or moderate in severity. Three deaths were reported. The treatment was stopped due to AEs in five patients (three treated with PrODR and two with LSR).
Conclusion: Efficacy and safety of treatment with PrODR and LSR is comparable in BOC or TVR-experienced patients.
Keywords: Chronic hepatitis C; Liver cirrhosis; Protease inhibitors; Retreatment; Sustained virologic response.
Conflict of interest statement
Ethics approval and consent to participate
This retrospective, observational study was carried out in real-life setting, with approved drugs. Patients were not exposed to any experimental interventions nor did the study intervene with the clinical management of the patient. The study only collected information from patient records. The analysis included routine examinations and tests performed in patients treated within the therapeutic program of the National Health Fund. The data was originally collected to assess treatment efficacy and safety in individual patient, not for scientific purposes. Hence, the treating physicians did not take approval from the ethics committee of any research/academic institute. According to local law (Pharmaceutical Law of 6th September 2001, art. 37al) non-interventional studies do not require approval of ethics committee. Patients signed informed consent for treatment and processing of personal data. Patients’ data were collected through an on-line system and only physicians who have patients in care had access to patients’ personal information.
Consent for publication
The manuscript doesn’t contain details, images, or videos relating to an individual person.
Competing interests
EJ has received research grants and/or fees for lectures, advisory boards, scientific consultancies from Abbvie, Allergan, Bristol Meyer Squibb, Gilead Sciences, Janssen Cilag, MSD, Vertex, Tobira. AP has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, Gilead, Merck, Roche. WM has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, BMS, Gilead, Janssen, Merck, Roche. TBM has received research grants and fees for scientific consultancies for: AbbVie, Gilead. WD has received fees for scientific consultancies from: AbbVie, Gilead. ACA has received research grants from: AbbVie, Merck. JJ has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, BMS, Gilead, Merz, Roche. ŁS has received fees for scientific consultancies from BMS. BA has received research grants from BMS and fees for scientific consultancies from Roche, AbbVie, BMS, Gilead, MSD. WH has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, BMS, Gilead, Janssen, Merck, Roche. BBC has received research grants and/or fees for lectures, scientific consultancies from: AbbVie, Gilead, Roche. KT has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, Alfa Wasserman, BMS, Gilead, Janssen, Merck, Roche. KS has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, Gilead, BMS, Merck, Janssen, Alfa-Wassermann, Baxter, Bayer, Roche Allergan, EISAI, Gilead, Intercept, Tobira, Pfizer.
AG has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Roche, Sanofi Pasteur, Amgen, Pfizer. MWS has received fees for lectures, scientific consultancies from: Gilead, AbbVie, Merck. RF has received research grants and/or fees for lectures, advisory boards, scientific consultancies from: AbbVie, Alfa Wasserman, BMS, Gilead, Janssen, Merck, Roche, Shionogi. DZM, HB, DD, MTZ, ZD, IB, MS, BL, JBW, JC, ŁL, OT, AH, AG declare no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kobayashi N, Iijima H, Tada T, Kumada T, Yoshida M, Aoki T, et al. Changes in liver stiffness and steatosis among patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Eur J Gastroenterol Hepatol. 2018;30:546–551. doi: 10.1097/MEG.0000000000001106. - DOI - PubMed
-
- Tada T, Kumada T, Toyoda H, Sone Y, Takeshima K, Ogawa S, et al. Viral eradication reduces both liver stiffness and steatosis in patients with chronic hepatitis C virus infection who received direct-acting anti-viral therapy. Aliment Pharmacol Ther. 2018;47:1012–1022. doi: 10.1111/apt.14554. - DOI - PubMed
-
- Kozbial Karin, Moser Stephan, Al-Zoairy Ramona, Schwarzer Remy, Datz Christian, Stauber Rudolf, Laferl Hermann, Strasser Michael, Beinhardt Sandra, Stättermayer Albert F., Gschwantler Michael, Zoller Heinz, Maieron Andreas, Graziadei Ivo, Trauner Michael, Steindl-Munda Petra, Hofer Harald, Ferenci Peter. Follow-up of sustained virological responders with hepatitis C and advanced liver disease after interferon/ribavirin-free treatment. Liver International. 2017;38(6):1028–1035. doi: 10.1111/liv.13629. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

