Human papillomavirus type 16 E6 and E7 oncoproteins interact with the nuclear p53-binding protein 1 in an in vitro reconstructed 3D epithelium: new insights for the virus-induced DNA damage response
- PMID: 30445982
- PMCID: PMC6240266
- DOI: 10.1186/s12985-018-1086-4
Human papillomavirus type 16 E6 and E7 oncoproteins interact with the nuclear p53-binding protein 1 in an in vitro reconstructed 3D epithelium: new insights for the virus-induced DNA damage response
Abstract
Background: Despite vaccination and screening measures, anogenital cancer, mainly promoted by HPV16 oncoproteins, still represents the fourth tumor and the second cause of death among women. Cell replication fidelity is the result of the host DNA damage response (DDR). Unlike many DNA viruses that promote their life cycle through the DDR inactivation, HR-HPVs encourage cells proliferation despite the DDR turned on. Why and how it occurs has been only partially elucidated. During HPV16 infection, E6 links and degrades p53 via the binding to the E6AP LXXLL sequence; unfortunately, E6 direct role in the DDR response has not clearly identified yet. Similarly, E7 increases DDR by competing with E2F1-pRb interaction, thus leading to the inactivation of pRb, and promotion, E2F1 mediated, of DDR genes translation, by binding to the pRb-like proteins CBP/p300 and p107, that also harbour LXXLL sequence, and via the interaction and activation of several DDR proteins.
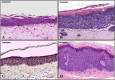
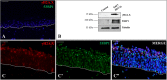
Methods: To gain information regarding E6 and E7 contribution in DDR activation, we produced an in vitro 3D HPV16-E6E7 infected epithelium, already consolidated study model for HPVs, and validated it by assessing H&E staining and BrdU, HPV16 DNA, E6E7 proteins and γH2A.X/53BP1 double-strand break (DSBs) sensors expression; then we made an immuno-colocalization of E6 and E7 with cyclin E2 and B1. Since 53BP1, like E6 and E7, also binds p53 and pRb, we supposed their possible direct binding. To explore this hypothesis, we performed a double immunofluorescence of E6 and E7 with 53BP1, a sequence analysis of 53BP1 within its BRCT2 domain and then an in situ PLA within CaSki, E6E7HPV16 NHEKs and the 3D model.
Results: The in vitro epithelium resembled the histology and the events typical of in vivo infected tissues. E6E7HPV16 were both expressed in basal and differentiated strata and induced H2A.X phosphorylation and 53BP1 increment into nuclear foci. After highlighting E6 and E7 co-expression with 53BP1 and a LKVLL sequence within the 53BP1 BRCT2 domain, we demonstrated the bindings via the PLA technique.
Conclusions: Our results reinforce E6 and E7 role in cellular function control providing potentially new insights into the activity of this tumor virus.
Keywords: Cancer; DNA damage response; Double-strand break; E6-associated protein; Genomic instability; High-risk Human Papillomavirus; In vitro 3D epithelial model; Proximity ligation assay; World Health Organization.
Conflict of interest statement
Ethics approval and consent to participate
An approval for this study (prot. n. 620/CE, study n. CE 40/14), carried out in accordance with the declaration of Helsinki as revised in 2013, was granted by the Hospital Research Ethics Committee, Novara, Italy.
Ethics Commitee board members: President - Roberto Fantozzi; Components - Gian Carlo Avanzi, Maria Angela Brustia, Claudio De Pieri, Roberto Fantozzi, Edoardo Ferlito, Lorenzo Giudice, Gianenrico Guida, Corrado Magnani, Francesco Pia, Alessia Pisterna, Pacifico Uglietti, Libero Zannino.
This manuscript does not involve the use of any animal.
Competing interests
The authors certify that they have no competing or conflict of interests relevant to the study to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Wood NH, Khammissa RA, Chikte UM, Meyerov R, Lemmer J, Feller L. The pathobiology and mechanisms of infection of HPV. SADJ. 2010;65:124–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

