STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial
- PMID: 30446007
- PMCID: PMC6240242
- DOI: 10.1186/s40425-018-0436-5
STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial
Abstract
Background: The Janus kinase (JAK) and signal transduction and activation of transcription (STAT) signaling pathway is an attractive target in multiple cancers. Activation of the JAK-STAT pathway is important in both tumorigenesis and activation of immune responses. In diffuse large B-cell lymphoma (DLBCL), the transcription factor STAT3 has been associated with aggressive disease phenotype and worse overall survival. While multiple therapies inhibit upstream signaling, there has been limited success in selectively targeting STAT3 in patients. Antisense oligonucleotides (ASOs) represent a compelling therapeutic approach to target difficult to drug proteins such as STAT3 through of mRNA targeting. We report the evaluation of a next generation STAT3 ASO (AZD9150) in a non-Hodgkin's lymphoma population, primarily consisting of patients with DLBCL.
Methods: Patients with relapsed or treatment refractory lymphoma were enrolled in this expansion cohort. AZD9150 was administered at 2 mg/kg and the 3 mg/kg (MTD determined by escalation cohort) dose levels with initial loading doses in the first week on days 1, 3, and 5 followed by weekly dosing. Patients were eligible to remain on therapy until unacceptable toxicity or progression. Blood was collected pre- and post-treatment for analysis of peripheral immune cells.
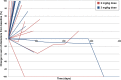
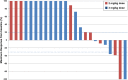
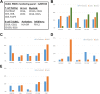
Results: Thirty patients were enrolled, 10 at 2 mg/kg and 20 at 3 mg/kg dose levels. Twenty-seven patients had DLBCL. AZD9150 was safe and well tolerated at both doses. Common drug-related adverse events included transaminitis, fatigue, and thrombocytopenia. The 3 mg/kg dose level is the recommended phase 2 dose. All responses were seen among DLBCL patients, including 2 complete responses with median duration of response 10.7 months and 2 partial responses. Peripheral blood cell analysis of three patients without a clinical response to therapy revealed a relative increase in proportion of macrophages, CD4+, and CD8+ T cells; this trend did not reach statistical significance.
Conclusions: AZD9150 was well tolerated and demonstrated efficacy in a subset of heavily pretreated patients with DLBCL. Studies in combination with checkpoint immunotherapies are ongoing.
Trial registration: Registered at ClinicalTrials.gov: NCT01563302 . First submitted 2/13/2012.
Keywords: Anti-sense oligonucleotide; Clinical trial; DLBCL; Diffuse large B-cell lymphoma; Immunotherapy; JAK-STAT; Lymphoma; STAT3.
Conflict of interest statement
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board at the University of Texas MD Anderson Cancer Center. Written informed consent was obtained from the patients prior to treatment.
Consent for publication
Written informed consent was obtained from the patients for publication of their individual details and accompanying images in this manuscript. The consent form is held by the authors and is available for review by the Editor-in-Chief.
Competing interests
PM, CC, PL, RW, and MVPN are employees of Astrazeneca. YK, TZ, JS, MJ, SJL, MY, SGH, XX, JH, AR, BPM, and ARM are employees of Ionis Pharmaceuticals. The remaining authors declare no potential conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science [Internet] 1994;264(5164):1415–1421. - PubMed
-
- Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science [Internet] 1994;264(5155):95–98. - PubMed
-
- Lütticken C, Wegenka UM, Yuan J, Buschmann J, Schindler C, Ziemiecki A, et al. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science [Internet] 1994;263(5143):89–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
