Polymeric fluorescent heparin as one-step FRET substrate of human heparanase
- PMID: 30446119
- PMCID: PMC6245667
- DOI: 10.1016/j.carbpol.2018.10.071
Polymeric fluorescent heparin as one-step FRET substrate of human heparanase
Abstract
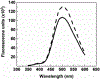
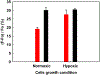
Heparanase, an endo-β-D-glucuronidase, cleaves cell surface and extracellular matrix heparan sulfate (HS) chains and plays important roles in cellular growth and metastasis. Heparanase assays reported to-date are labor intensive, complex and/or expensive. A simpler assay is critically needed to understand the myriad roles of heparanase. We reasoned that fluorescent heparin could serve as an effective probe of heparanase levels. Following synthesis and screening, a heparin preparation labeled with DABCYL and EDANS was identified, which exhibited a characteristic increase in signal following cleavage by human heparanase. This work describes the synthesis of this heparin substrate, its kinetic and spectrofluorometric properties, optimization of the heparanase assay, use of the assay in inhibitor screening, and elucidation of the state of heparanase in different cell lines. Our FRET-based assay is much simpler and more robust than all assays reported in the literature as well as a commercially available kit.
Keywords: Enzyme assay; Enzyme inhibition; FRET; Fluorescent heparin; Heparanase; Heparin.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
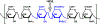



Similar articles
-
A Robust, One-step FRET Assay for Human Heparanase.Bio Protoc. 2019 Sep 5;9(17):e3356. doi: 10.21769/BioProtoc.3356. eCollection 2019 Sep 5. Bio Protoc. 2019. PMID: 33654855 Free PMC article.
-
Kinetic analysis and molecular modeling of the inhibition mechanism of roneparstat (SST0001) on human heparanase.Glycobiology. 2016 Jun;26(6):640-54. doi: 10.1093/glycob/cww003. Epub 2016 Jan 13. Glycobiology. 2016. PMID: 26762172 Free PMC article.
-
Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting.J Biol Chem. 2005 Apr 1;280(13):12103-13. doi: 10.1074/jbc.M414217200. Epub 2005 Jan 12. J Biol Chem. 2005. PMID: 15647251
-
Molecular Aspects of Heparanase Interaction with Heparan Sulfate, Heparin and Glycol Split Heparin.Adv Exp Med Biol. 2020;1221:169-188. doi: 10.1007/978-3-030-34521-1_6. Adv Exp Med Biol. 2020. PMID: 32274710 Review.
-
Heparanase, heparin and the coagulation system in cancer progression.Thromb Res. 2007;120 Suppl 2:S112-20. doi: 10.1016/S0049-3848(07)70139-1. Thromb Res. 2007. PMID: 18023704 Review.
Cited by
-
A Robust, One-step FRET Assay for Human Heparanase.Bio Protoc. 2019 Sep 5;9(17):e3356. doi: 10.21769/BioProtoc.3356. eCollection 2019 Sep 5. Bio Protoc. 2019. PMID: 33654855 Free PMC article.
-
Heparanase expression and activity are increased in platelets during clinical sepsis.J Thromb Haemost. 2021 May;19(5):1319-1330. doi: 10.1111/jth.15266. Epub 2021 Mar 11. J Thromb Haemost. 2021. PMID: 33587773 Free PMC article.
-
Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation.Int J Mol Sci. 2019 Apr 22;20(8):1963. doi: 10.3390/ijms20081963. Int J Mol Sci. 2019. PMID: 31013618 Free PMC article. Review.
-
Synthetic Strategies for FRET-Enabled Carbohydrate Active Enzyme Probes.Methods Mol Biol. 2022;2370:237-264. doi: 10.1007/978-1-0716-1685-7_12. Methods Mol Biol. 2022. PMID: 34611873
-
COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction.JCI Insight. 2021 Sep 8;6(17):e147472. doi: 10.1172/jci.insight.147472. JCI Insight. 2021. PMID: 34314391 Free PMC article.
References
-
- Allen MD, & Zhang J (2006). Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochemical and Biophysical Research Communications, 348(2), 716–721. https://doi.org/10.1016/j.bbrc.2006.07.136 - DOI - PubMed
-
- Behzad F, & Brenchley PEC (2003). A multiwell format assay for heparanase. Analytical Biochemistry, 320(2), 207–213. https://doi.org/10.1016/S0003-2697(03)00358-0 - DOI - PubMed
-
- Bhushan I, Alabbas A, Kuberan B, Gupta RB, & Desai UR (2017). Immobilization alters heparin cleaving properties of heparinase I. Glycobiology, (October), 1–5. https://doi.org/10.1093/glycob/cwx074 - DOI - PMC - PubMed
-
- Chen L, & Sanderson RD (2009). Heparanase regulates levels of syndecan-1 in the nucleus. PLoS ONE, 4(3), 1–6. https://doi.org/10.1371/journal.pone.0004947 - DOI - PMC - PubMed
-
- David G, & Zimmermann P (2016). Heparanase tailors syndecan for exosome production. Molecular & Cellular Oncology, 3(3), el047556 https://doi.org/10.1080/23723556.2015.1047556 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

