Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis
- PMID: 30446388
- PMCID: PMC6303217
- DOI: 10.1016/j.immuni.2018.09.018
Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis
Abstract
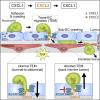
Neutrophils require directional cues to navigate through the complex structure of venular walls and into inflamed tissues. Here we applied confocal intravital microscopy to analyze neutrophil emigration in cytokine-stimulated mouse cremaster muscles. We identified differential and non-redundant roles for the chemokines CXCL1 and CXCL2, governed by their distinct cellular sources. CXCL1 was produced mainly by TNF-stimulated endothelial cells (ECs) and pericytes and supported luminal and sub-EC neutrophil crawling. Conversely, neutrophils were the main producers of CXCL2, and this chemokine was critical for correct breaching of endothelial junctions. This pro-migratory activity of CXCL2 depended on the atypical chemokine receptor 1 (ACKR1), which is enriched within endothelial junctions. Transmigrating neutrophils promoted a self-guided migration response through EC junctions, creating a junctional chemokine "depot" in the form of ACKR1-presented CXCL2 that enabled efficient unidirectional luminal-to-abluminal migration. Thus, CXCL1 and CXCL2 act in a sequential manner to guide neutrophils through venular walls as governed by their distinct cellular sources.
Keywords: ACKR1; CXCR2; chemokines; endothelium; extravasation; inflammation; neutrophils; pericytes.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
Neutrophils Stepping Through (to the Other Side).Immunity. 2018 Dec 18;49(6):992-994. doi: 10.1016/j.immuni.2018.12.006. Immunity. 2018. PMID: 30566887
References
-
- Armstrong D.A., Major J.A., Chudyk A., Hamilton T.A. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J. Leukoc. Biol. 2004;75:641–648. - PubMed
-
- Cacalano G., Lee J., Kikly K., Ryan A.M., Pitts-Meek S., Hultgren B., Wood W.I., Moore M.W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. - PubMed
-
- Dawson T.C., Lentsch A.B., Wang Z., Cowhig J.E., Rot A., Maeda N., Peiper S.C. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

