Dopamine Restores Limbic Memory Loss, Dendritic Spine Structure, and NMDAR-Dependent LTD in the Nucleus Accumbens of Alcohol-Withdrawn Rats
- PMID: 30446531
- PMCID: PMC6382989
- DOI: 10.1523/JNEUROSCI.1377-18.2018
Dopamine Restores Limbic Memory Loss, Dendritic Spine Structure, and NMDAR-Dependent LTD in the Nucleus Accumbens of Alcohol-Withdrawn Rats
Abstract
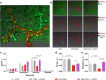
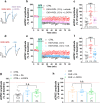
Alcohol abuse leads to aberrant forms of emotionally salient memory, i.e., limbic memory, that promote escalated alcohol consumption and relapse. Accordingly, activity-dependent structural abnormalities are likely to contribute to synaptic dysfunctions that occur from suddenly ceasing chronic alcohol consumption. Here we show that alcohol-dependent male rats fail to perform an emotional-learning task during abstinence but recover their functioning by l-3,4-dihydroxyphenylalanin (l-DOPA) administration during early withdrawal. l-DOPA also reverses the selective loss of dendritic "long thin" spines observed in medium spiny neurons of the nucleus accumbens (NAc) shell of alcohol-dependent rats during abstinence, as well as the reduction in tyrosine hydroxylase immunostaining and postsynaptic density-95-positive elements. Patch-clamp experiments in NAc slices reveal that both in vivo systemic l-DOPA administration and in vitro exposure to dopamine can restore the loss of long-term depression (LTD) formation, counteract the reduction in NMDAR-mediated synaptic currents and rectify the altered NMDAR/AMPAR ratio observed in alcohol-withdrawn rats. Further, in vivo microdialysis experiments show that blunted dopaminergic signaling is revived after l-DOPA treatment during early withdrawal. These results suggest a key role of an efficient dopamine signaling for maintaining, and restore, neural trophism, NMDA-dependent LTD, and ultimately optimal learning.SIGNIFICANCE STATEMENT Blunted dopamine signaling and altered glutamate connectivity in the nucleus accumbens represent the neuroanatomical basis for the impairment in aversive limbic memory observed during withdrawal in alcohol dependence. Supplying l-DOPA during withdrawal re-establishes synaptic morphology and functional neuroadaptations, suggesting a complete recovery of nucleus accumbens glutamatergic synaptic plasticity when dopamine is revived. Importantly, restoring dopamine transmission allows those synapses to encode emotionally relevant information and rescue flexibility in the neuronal circuits that process limbic memory formation. Under these conditions, drugs capable of selectively boosting the dopaminergic function during the "fluid" and still responsive state of the early withdrawn maladaptive synapses may help in the treatment of alcohol addiction.
Keywords: LTD; alcohol abuse; confocal microscopy; dopamine; glutamate; memory.
Copyright © 2019 the authors 0270-6474/19/390930-15$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
