Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse
- PMID: 30446532
- PMCID: PMC6335749
- DOI: 10.1523/JNEUROSCI.0537-18.2018
Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse
Abstract
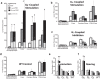
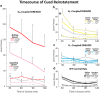
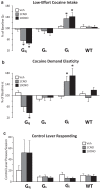
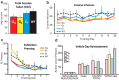
Ventral tegmental area (VTA) dopamine (DA) neurons perform diverse functions in motivation and cognition, but their precise roles in addiction-related behaviors are still debated. Here, we targeted VTA DA neurons for bidirectional chemogenetic modulation during specific tests of cocaine reinforcement, demand, and relapse-related behaviors in male rats, querying the roles of DA neuron inhibitory and excitatory G-protein signaling in these processes. Designer receptor stimulation of Gq signaling, but not Gs signaling, in DA neurons enhanced cocaine seeking via functionally distinct projections to forebrain limbic regions. In contrast, engaging inhibitory Gi/o signaling in DA neurons blunted the reinforcing and priming effects of cocaine, reduced stress-potentiated reinstatement, and altered behavioral strategies for cocaine seeking and taking. Results demonstrate that DA neurons play several distinct roles in cocaine seeking, depending on behavioral context, G-protein-signaling cascades, and DA neuron efferent targets, highlighting their multifaceted roles in addiction.SIGNIFICANCE STATEMENT G-protein-coupled receptors are crucial modulators of ventral tegmental area (VTA) dopamine neuron activity, but how this metabotropic signaling impacts the complex roles of dopamine in reward and addiction is poorly understood. Here, we bidirectionally modulate dopamine neuron G-protein signaling with DREADDs (designer receptors exclusively activated by designer drugs) during a variety of cocaine-seeking behaviors, revealing nuanced, pathway-specific roles in cocaine reward, effortful seeking, and relapse-like behaviors. Gq and Gs stimulation activated dopamine neurons, but only Gq stimulation robustly enhanced cocaine seeking. Gi/o inhibitory signaling reduced some, but not all, types of cocaine seeking. Results show that VTA dopamine neurons modulate numerous distinct aspects of cocaine addiction- and relapse-related behaviors, and point to potential new approaches for intervening in these processes to treat addiction.
Keywords: DREADDs; addiction; conditioned cues; motivation; neural circuits; reinstatement.
Copyright © 2019 the authors 0270-6474/19/390503-16$15.00/0.
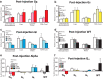
Figures



References
-
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L (2011) Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci 31:10829–10835. 10.1523/JNEUROSCI.2246-11.2011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 DA036989/DA/NIDA NIH HHS/United States
- R01 DA031900/DA/NIDA NIH HHS/United States
- R01 DA033342/DA/NIDA NIH HHS/United States
- P50 DA015369/DA/NIDA NIH HHS/United States
- R01 DA046476/DA/NIDA NIH HHS/United States
- F31 DA042505/DA/NIDA NIH HHS/United States
- R00 DA035251/DA/NIDA NIH HHS/United States
- T32 DA007288/DA/NIDA NIH HHS/United States
- P50 DA044118/DA/NIDA NIH HHS/United States
- TL1 TR001415/TR/NCATS NIH HHS/United States
- R01 DA006214/DA/NIDA NIH HHS/United States
- R00 DA040004/DA/NIDA NIH HHS/United States
- K99 DA035251/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
