A Proposed Mechanism for Spontaneous Transitions between Interictal and Ictal Activity
- PMID: 30446533
- PMCID: PMC6335741
- DOI: 10.1523/JNEUROSCI.0719-17.2018
A Proposed Mechanism for Spontaneous Transitions between Interictal and Ictal Activity
Abstract
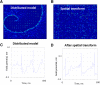
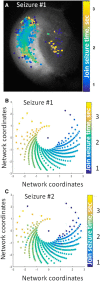
Epileptic networks are characterized by two outputs: brief interictal spikes and rarer, more prolonged seizures. Although either output state is readily modeled in silico and induced experimentally, the transition mechanisms are unknown, in part because no models exhibit both output states spontaneously. In silico small-world neural networks were built using single-compartment neurons whose physiological parameters were derived from dual whole-cell recordings of pyramidal cells in organotypic hippocampal slice cultures that were generating spontaneous seizure-like activity. In silico, neurons were connected by abundant local synapses and rare long-distance synapses. Activity-dependent synaptic depression and gradual recovery delimited synchronous activity. Full synaptic recovery engendered interictal population spikes that spread via long-distance synapses. When synaptic recovery was incomplete, postsynaptic neurons required coincident activation of multiple presynaptic terminals to reach firing threshold. Only local connections were sufficiently dense to spread activity under these conditions. This coalesced network activity into traveling waves whose velocity varied with synaptic recovery. Seizures were comprised of sustained traveling waves that were similar to those recorded during experimental and human neocortical seizures. Sustained traveling waves occurred only when wave velocity, network dimensions, and the rate of synaptic recovery enabled wave reentry into previously depressed areas at precisely ictogenic levels of synaptic recovery. Wide-field, cellular-resolution GCamP7b calcium imaging demonstrated similar initial patterns of activation in the hippocampus, although the anatomical distribution of traveling waves of synaptic activation was altered by the pattern of synaptic connectivity in the organotypic hippocampal cultures.SIGNIFICANCE STATEMENT When computerized distributed neural network models are required to generate both features of epileptic networks (i.e., spontaneous interictal population spikes and seizures), the network structure is substantially constrained. These constraints provide important new hypotheses regarding the nature of epileptic networks and mechanisms of seizure onset.
Keywords: epilepsy; ictogenesis; model; propagation; seizure; wavefront.
Copyright © 2019 the authors 0270-6474/19/390557-19$15.00/0.
Figures










Comment in
-
Goldilocks Zone of Ictal Onset: Partially Recovered Synapses Provide the Kindling to Fuel Ictal Activity.Epilepsy Curr. 2019 Sep;19(5):330-332. doi: 10.1177/1535759719869670. Epub 2019 Aug 13. Epilepsy Curr. 2019. PMID: 31409150 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
