Object features and T4/T5 motion detectors modulate the dynamics of bar tracking by Drosophila
- PMID: 30446539
- PMCID: PMC6361104
- DOI: 10.1242/jeb.190017
Object features and T4/T5 motion detectors modulate the dynamics of bar tracking by Drosophila
Abstract
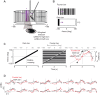
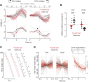
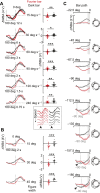
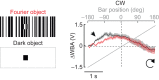
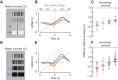
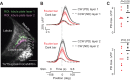
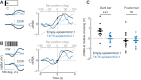
Visual objects can be discriminated by static spatial features such as luminance or dynamic features such as relative movement. Flies track a solid dark vertical bar moving on a bright background, a behavioral reaction so strong that for a rigidly tethered fly, the steering trajectory is phase advanced relative to the moving bar, apparently in anticipation of its future position. By contrast, flickering bars that generate no coherent motion or have a surface texture that moves in the direction opposite to the bar generate steering responses that lag behind the stimulus. It remains unclear how the spatial properties of a bar influence behavioral response dynamics. Here, we show that a dark bar defined by its luminance contrast to the uniform background drives a co-directional steering response that is phase advanced relative to the response to a textured bar defined only by its motion relative to a stationary textured background. The textured bar drives an initial contra-directional turn and phase-locked tracking. The qualitatively distinct response dynamics could indicate parallel visual processing of a luminance versus motion-defined object. Calcium imaging shows that T4/T5 motion-detecting neurons are more responsive to a solid dark bar than a motion-defined bar. Genetically blocking T4/T5 neurons eliminates the phase-advanced co-directional response to the luminance-defined bar, leaving the orientation response largely intact. We conclude that T4/T5 neurons mediate a co-directional optomotor response to a luminance-defined bar, thereby driving phase-advanced wing kinematics, whereas separate unknown visual pathways elicit the contra-directional orientation response.
Keywords: Feature detection; Fly flight; Motion vision; Tethered flight; Visual behavior.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Columnar neurons support saccadic bar tracking in Drosophila.Elife. 2023 Apr 4;12:e83656. doi: 10.7554/eLife.83656. Elife. 2023. PMID: 37014060 Free PMC article.
-
A Visual Pathway into Central Complex for High-Frequency Motion-Defined Bars in Drosophila.J Neurosci. 2023 Jun 28;43(26):4821-4836. doi: 10.1523/JNEUROSCI.0128-23.2023. Epub 2023 Jun 8. J Neurosci. 2023. PMID: 37290936 Free PMC article.
-
A directional tuning map of Drosophila elementary motion detectors.Nature. 2013 Aug 8;500(7461):212-6. doi: 10.1038/nature12320. Nature. 2013. PMID: 23925246
-
How fly neurons compute the direction of visual motion.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020 Mar;206(2):109-124. doi: 10.1007/s00359-019-01375-9. Epub 2019 Nov 5. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2020. PMID: 31691093 Free PMC article. Review.
-
In search of the Holy Grail of fly motion vision.Eur J Neurosci. 2014 Nov;40(9):3285-93. doi: 10.1111/ejn.12731. Epub 2014 Sep 23. Eur J Neurosci. 2014. PMID: 25251169 Review.
Cited by
-
The influence of saccades on yaw gaze stabilization in fly flight.PLoS Comput Biol. 2023 Dec 21;19(12):e1011746. doi: 10.1371/journal.pcbi.1011746. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38127819 Free PMC article.
-
Columnar neurons support saccadic bar tracking in Drosophila.Elife. 2023 Apr 4;12:e83656. doi: 10.7554/eLife.83656. Elife. 2023. PMID: 37014060 Free PMC article.
-
Fly eyes are not still: a motion illusion in Drosophila flight supports parallel visual processing.J Exp Biol. 2020 May 28;223(Pt 10):jeb212316. doi: 10.1242/jeb.212316. J Exp Biol. 2020. PMID: 32321749 Free PMC article.
-
Olfactory and Neuromodulatory Signals Reverse Visual Object Avoidance to Approach in Drosophila.Curr Biol. 2019 Jun 17;29(12):2058-2065.e2. doi: 10.1016/j.cub.2019.05.010. Epub 2019 May 30. Curr Biol. 2019. PMID: 31155354 Free PMC article.
-
Mosquitoes integrate visual and acoustic cues to mediate conspecific interactions in swarms.Curr Biol. 2024 Sep 23;34(18):4091-4103.e4. doi: 10.1016/j.cub.2024.07.043. Epub 2024 Aug 30. Curr Biol. 2024. PMID: 39216484
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

