Sialic Acid-Dependent Inhibition of T Cells by Exosomal Ganglioside GD3 in Ovarian Tumor Microenvironments
- PMID: 30446565
- PMCID: PMC6289713
- DOI: 10.4049/jimmunol.1801041
Sialic Acid-Dependent Inhibition of T Cells by Exosomal Ganglioside GD3 in Ovarian Tumor Microenvironments
Abstract
The tumor microenvironment is rendered immunosuppressive by a variety of cellular and acellular factors that represent potential cancer therapeutic targets. Although exosomes isolated from ovarian tumor ascites fluids have been previously reported to induce a rapid and reversible T cell arrest, the factors present on or within exosomes that contribute to immunosuppression have not been fully defined. In this study, we establish that GD3, a ganglioside expressed on the surface of exosomes isolated from human ovarian tumor ascites fluids, is causally linked to the functional arrest of T cells activated through their TCR. This arrest is inhibited by Ab blockade of exosomal GD3 or by the removal of GD3+ exosomes. Empty liposomes expressing GD3 on the surface also inhibit the activation of T cells, establishing that GD3 contributes to the functional arrest of T cells independent of factors present in exosomes. Finally, we demonstrate that the GD3-mediated arrest of the TCR activation is dependent upon sialic acid groups, because their enzymatic removal from exosomes or liposomes results in a loss of inhibitory capacity. Collectively, these data define GD3 as a potential immunotherapeutic target.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures
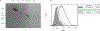
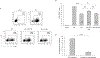
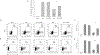

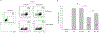

References
-
- Santin AD, Bellone S, Ravaggi A, Pecorelli S, Cannon MJ, and Parham GP 2000. Induction of ovarian tumor-specific CD8+ cytotoxic T lymphocytes by acid-eluted peptide-pulsed autologous dendritic cells. Obstet Gynecol 96: 422–430. - PubMed
-
- Ramakrishna V, Ross MM, Petersson M, Gatlin CC, Lyons CE, Miller CL, Myers HE, McDaniel M, Karns LR, Kiessling R, Parmiani G, and Flyer DC 2003. Naturally occurring peptides associated with HLA-A2 in ovarian cancer cell lines identified by mass spectrometry are targets of HLA-A2-restricted cytotoxic T cells. Int Immunol 15: 751–763. - PubMed
-
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, and Coukos G 2003. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348: 203–213. - PubMed
-
- Yokota SJ, Facciponte JG, Kelleher RJ Jr., Shultz LD, Loyall JL, Parsons RR, Odunsi K, Frelinger JG, Lord EM, Gerber SA, Balu-Iyer SV, and Bankert RB 2013. Changes in ovarian tumor cell number, tumor vasculature, and T cell function monitored in vivo using a novel xenograft model. Cancer Immun 13: 11. - PMC - PubMed
-
- Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, June CH, Riley JL, Wherry EJ, and Albelda SM 2014. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20: 4262–4273. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

