AKT Inhibition Modulates H3K4 Demethylase Levels in PTEN-Null Prostate Cancer
- PMID: 30446585
- PMCID: PMC8488980
- DOI: 10.1158/1535-7163.MCT-18-0141
AKT Inhibition Modulates H3K4 Demethylase Levels in PTEN-Null Prostate Cancer
Abstract
Hyperactivated AKT kinase due to loss of its negative regulator PTEN influences many aspects of cancer biology, including chromatin. AKT primarily regulates acetyl-CoA production and phosphorylates many histone-modulating enzymes, resulting in their activation or inhibition. Therefore, understanding the therapeutic impact of AKT inhibition on chromatin-related events is essential. Here, we report that AKT inhibition in prostate-specific PTEN knockout mice significantly induces di- and trimethylation of H3K4 with concomitant reduction in H3K9 acetylation. Mechanistically, we observed that AKT inhibition reduces expression of the H3K4 methylation-specific histone demethylases KDM5 family, especially KDM5B expression at transcriptional levels. Furthermore, we observed that AKT negatively regulates miR-137 levels, which transcriptionally represses KDM5B expression. Overexpression of miR-137 significantly reduced KDM5B and increased H3K4 methylation levels but failed to change AKT phosphorylation. Overall, we observed that AKT transcriptionally regulates KDM5B mainly via repression of miR-137. Our data identify a mechanism by which AKT kinase modulates the prostate cancer epigenome through regulating H3K4 methylation. Additional studies on AKT inhibition-mediated induction of H3K4 methylation will help in designing strategies to enhance the therapeutic efficacy of PI3K/AKT inhibitors.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures

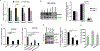
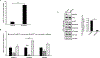
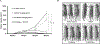
Similar articles
-
KDM5B Is Essential for the Hyperactivation of PI3K/AKT Signaling in Prostate Tumorigenesis.Cancer Res. 2020 Nov 1;80(21):4633-4643. doi: 10.1158/0008-5472.CAN-20-0505. Epub 2020 Aug 31. Cancer Res. 2020. PMID: 32868382 Free PMC article.
-
KDM5B demethylates H3K4 to recruit XRCC1 and promote chemoresistance.Int J Biol Sci. 2018 Jun 22;14(9):1122-1132. doi: 10.7150/ijbs.25881. eCollection 2018. Int J Biol Sci. 2018. PMID: 29989047 Free PMC article.
-
KDM5B focuses H3K4 methylation near promoters and enhancers during embryonic stem cell self-renewal and differentiation.Genome Biol. 2014 Feb 4;15(2):R32. doi: 10.1186/gb-2014-15-2-r32. Genome Biol. 2014. PMID: 24495580 Free PMC article.
-
KDM5B is a master regulator of the H3K4-methylome in stem cells, development and cancer.Semin Cancer Biol. 2019 Aug;57:79-85. doi: 10.1016/j.semcancer.2018.11.001. Epub 2018 Nov 16. Semin Cancer Biol. 2019. PMID: 30448242 Free PMC article. Review.
-
Histone demethylase lysine demethylase 5B in development and cancer.Oncotarget. 2017 Jan 31;8(5):8980-8991. doi: 10.18632/oncotarget.13858. Oncotarget. 2017. PMID: 27974677 Free PMC article. Review.
Cited by
-
H3 histone methylation landscape in male urogenital cancers: from molecular mechanisms to epigenetic biomarkers and therapeutic targets.Front Cell Dev Biol. 2023 May 9;11:1181764. doi: 10.3389/fcell.2023.1181764. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37228649 Free PMC article. Review.
-
Lysine demethylase 5A promotes prostate adenocarcinoma progression by suppressing microRNA-330-3p expression and activating the COPB2/PI3K/AKT axis in an ETS1-dependent manner.J Cell Commun Signal. 2022 Dec;16(4):579-599. doi: 10.1007/s12079-022-00671-5. Epub 2022 May 18. J Cell Commun Signal. 2022. PMID: 35581421 Free PMC article.
-
DNA Methyltransferase 1 Targeting Using Guadecitabine Inhibits Prostate Cancer Growth by an Apoptosis-Independent Pathway.Cancers (Basel). 2023 May 15;15(10):2763. doi: 10.3390/cancers15102763. Cancers (Basel). 2023. PMID: 37345101 Free PMC article.
-
Diverse Functions of KDM5 in Cancer: Transcriptional Repressor or Activator?Cancers (Basel). 2022 Jul 4;14(13):3270. doi: 10.3390/cancers14133270. Cancers (Basel). 2022. PMID: 35805040 Free PMC article. Review.
-
Exploring the roles of ncRNAs in prostate cancer via the PI3K/AKT/mTOR signaling pathway.Front Immunol. 2025 Mar 18;16:1525741. doi: 10.3389/fimmu.2025.1525741. eCollection 2025. Front Immunol. 2025. PMID: 40170845 Free PMC article. Review.
References
-
- Potapova IA, El-Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry 2000;39:1169–79. - PubMed
-
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem 2002;277:33895–900. - PubMed
-
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, et al.Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 2005;310:306–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

