Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study
- PMID: 30446596
- PMCID: PMC6329331
- DOI: 10.1212/WNL.0000000000006640
Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study
Abstract
Objective: To evaluate the efficacy and safety of galcanezumab, a humanized monoclonal antibody that selectively binds to calcitonin gene-related peptide, in the preventive treatment of chronic migraine.
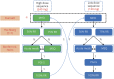
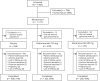
Methods: A phase 3, randomized, double-blind, placebo-controlled study of LY2951742 in patients with chronic migraine (Evaluation of Galcanezumab in the Prevention of Chronic Migraine [REGAIN]) was a phase 3 study with a 3-month double-blind, placebo-controlled treatment phase and a 9-month open-label extension. Eligible patients 18 to 65 years of age with chronic migraine were randomized 2:1:1 to monthly subcutaneous injections of placebo (n = 558), galcanezumab 120 mg (with a 240-mg loading dose, n = 278), or galcanezumab 240 mg (n = 277). The primary endpoint was the overall mean change from baseline in the number of monthly migraine headache days (MHDs) during the 3-month double-blind treatment phase.
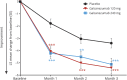
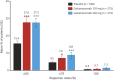
Results: Mean number of monthly MHDs at baseline was 19.4 for the total sample. Both galcanezumab dose groups demonstrated greater overall mean reduction in the number of monthly MHDs compared to placebo (placebo -2.7, galcanezumab 120 mg -4.8, galcanezumab 240 mg -4.6) (p < 0.001 for each dose compared to placebo). There were no clinically meaningful differences between galcanezumab doses and placebo on any safety or tolerability outcome except for a higher incidence of treatment-emergent injection-site reaction (p < 0.01), injection-site erythema (p < 0.001), injection-site pruritus (p < 0.01), and sinusitis (p < 0.05) in the galcanezumab 240-mg group relative to placebo.
Conclusions: Both doses of galcanezumab were superior to placebo in reducing the number of monthly MHDs. Galcanezumab appears efficacious, safe, and well tolerated for the preventive treatment of chronic migraine.
Clinicaltrialsgov identifier: NCT02614261.
Classification of evidence: This interventional study provides Class I evidence that galcanezumab is superior to placebo in the reduction of the number of monthly MHDs.
Copyright © 2018 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. - PubMed
-
- Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 2010;81:428–432. - PubMed
-
- Bigal ME, Lipton RB. Migraine chronification. Curr Neurol Neurosci Rep 2011;11:139–148. - PubMed
-
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 2010;6:573–582. - PubMed
-
- Smith J, Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Hirman J. Randomized, double-blind, placebo-controlled trial of ALD403 (eptinezumab), an anti-CGRP monoclonal antibody for the prevention of chronic migraine: 59th Annual Scientific Meeting American Headache Society® June 8–11, 2017 Westin Boston Waterfront Boston, MA. Headache 2017;57:130.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
