Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70
- PMID: 30446621
- PMCID: PMC6333884
- DOI: 10.1074/jbc.RA118.005536
Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70
Abstract
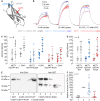
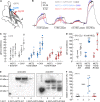
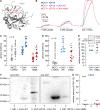
Monocarboxylate transporters (MCTs) mediate the proton-coupled exchange of high-energy metabolites, including lactate and pyruvate, between cells and tissues. The transport activity of MCT1, MCT2, and MCT4 can be facilitated by the extracellular carbonic anhydrase IV (CAIV) via a noncatalytic mechanism. Combining physiological measurements in HEK-293 cells and Xenopus oocytes with pulldown experiments, we analyzed the direct interaction between CAIV and the two MCT chaperones basigin (CD147) and embigin (GP70). Our results show that facilitation of MCT transport activity requires direct binding of CAIV to the transporters chaperones. We found that this binding is mediated by the highly conserved His-88 residue in CAIV, which is also the central residue of the enzyme's intramolecular proton shuttle, and a charged amino acid residue in the Ig1 domain of the chaperone. Although the position of the CAIV-binding site in the chaperone was conserved, the amino acid residue itself varied among different species. In human CD147, binding of CAIV was mediated by the negatively charged Glu-73 and in rat CD147 by the positively charged Lys-73. In rat GP70, we identified the positively charged Arg-130 as the binding site. Further analysis of the CAIV-binding site revealed that the His-88 in CAIV can either act as H donor or H acceptor for the hydrogen bond, depending on the charge of the binding residue in the chaperone. Our results suggest that the CAIV-mediated increase in MCT transport activity requires direct binding between CAIV-His-88 and a charged amino acid in the extracellular domain of the transporter's chaperone.
Keywords: Xenopus; electrophysiology; energy metabolism; ion-sensitive electrode; lactic acid; membrane transport; pH regulation; protein complex; protein expression; proton transport.
© 2019 Forero-Quintero et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






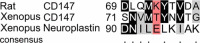
References
-
- Bröer S., Rahman B., Pellegri G., Pellerin L., Martin J. L., Verleysdonk S., Hamprecht B., and Magistretti P. J. (1997) Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 272, 30096–30102 10.1074/jbc.272.48.30096 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

