Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1
- PMID: 30446636
- PMCID: PMC5802550
- DOI: 10.1038/s41398-017-0054-x
Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1
Abstract
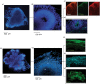
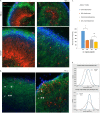
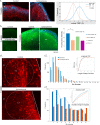
Studies of induced pluripotent stem cells (iPSCs) from schizophrenia patients and control individuals revealed that the disorder is programmed at the preneuronal stage, involves a common dysregulated mRNA transcriptome, and identified Integrative Nuclear FGFR1 Signaling a common dysregulated mechanism. We used human embryonic stem cell (hESC) and iPSC-derived cerebral organoids from four controls and three schizophrenia patients to model the first trimester of in utero brain development. The schizophrenia organoids revealed an abnormal scattering of proliferating Ki67+ neural progenitor cells (NPCs) from the ventricular zone (VZ), throughout the intermediate (IZ) and cortical (CZ) zones. TBR1 pioneer neurons and reelin, which guides cortico-petal migration, were restricted from the schizophrenia cortex. The maturing neurons were abundantly developed in the subcortical regions, but were depleted from the schizophrenia cortex. The decreased intracortical connectivity was denoted by changes in the orientation and morphology of calretinin interneurons. In schizophrenia organoids, nuclear (n)FGFR1 was abundantly expressed by developing subcortical cells, but was depleted from the neuronal committed cells (NCCs) of the CZ. Transfection of dominant negative and constitutively active nFGFR1 caused widespread disruption of the neuro-ontogenic gene networks in hESC-derived NPCs and NCCs. The fgfr1 gene was the most prominent FGFR gene expressed in NPCs and NCCs, and blocking with PD173074 reproduced both the loss of nFGFR1 and cortical neuronal maturation in hESC cerebral organoids. We report for the first time, progression of the cortical malformation in schizophrenia and link it to altered FGFR1 signaling. Targeting INFS may offer a preventive treatment of schizophrenia.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures





References
-
- Bogerts B, Hantsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol. Psychiatry. 1983;18:951–969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

