Cellular metabolism constrains innate immune responses in early human ontogeny
- PMID: 30446641
- PMCID: PMC6240060
- DOI: 10.1038/s41467-018-07215-9
Cellular metabolism constrains innate immune responses in early human ontogeny
Abstract
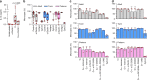
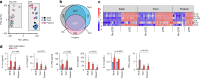
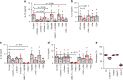
Pathogen immune responses are profoundly attenuated in fetuses and premature infants, yet the mechanisms underlying this developmental immaturity remain unclear. Here we show transcriptomic, metabolic and polysome profiling and find that monocytes isolated from infants born early in gestation display perturbations in PPAR-γ-regulated metabolic pathways, limited glycolytic capacity and reduced ribosomal activity. These metabolic changes are linked to a lack of translation of most cytokines and of MALT1 signalosome genes essential to respond to the neonatal pathogen Candida. In contrast, they have little impact on house-keeping phagocytosis functions. Transcriptome analyses further indicate a role for mTOR and its putative negative regulator DNA Damage Inducible Transcript 4-Like in regulating these metabolic constraints. Our results provide a molecular basis for the broad susceptibility to multiple pathogens in these infants, and suggest that the fetal immune system is metabolically programmed to avoid energetically costly, dispensable and potentially harmful immune responses during ontogeny.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

