Serotonin signals through a gut-liver axis to regulate hepatic steatosis
- PMID: 30446669
- PMCID: PMC6240035
- DOI: 10.1038/s41467-018-07287-7
Serotonin signals through a gut-liver axis to regulate hepatic steatosis
Erratum in
-
Publisher Correction: Serotonin signals through a gut-liver axis to regulate hepatic steatosis.Nat Commun. 2019 Jan 8;10(1):158. doi: 10.1038/s41467-018-08085-x. Nat Commun. 2019. PMID: 30622275 Free PMC article.
Abstract
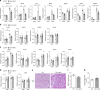
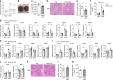
Nonalcoholic fatty liver disease (NAFLD) is increasing in worldwide prevalence, closely tracking the obesity epidemic, but specific pharmaceutical treatments for NAFLD are lacking. Defining the key molecular pathways underlying the pathogenesis of NAFLD is essential for developing new drugs. Here we demonstrate that inhibition of gut-derived serotonin synthesis ameliorates hepatic steatosis through a reduction in liver serotonin receptor 2A (HTR2A) signaling. Local serotonin concentrations in the portal blood, which can directly travel to and affect the liver, are selectively increased by high-fat diet (HFD) feeding in mice. Both gut-specific Tph1 knockout mice and liver-specific Htr2a knockout mice are resistant to HFD-induced hepatic steatosis, without affecting systemic energy homeostasis. Moreover, selective HTR2A antagonist treatment prevents HFD-induced hepatic steatosis. Thus, the gut TPH1-liver HTR2A axis shows promise as a drug target to ameliorate NAFLD with minimal systemic metabolic effects.
Conflict of interest statement
The authors declare no competing interests.
Figures



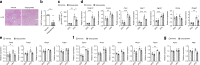
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

