Cued reactivation during slow-wave sleep induces brain connectivity changes related to memory stabilization
- PMID: 30446718
- PMCID: PMC6240046
- DOI: 10.1038/s41598-018-35287-6
Cued reactivation during slow-wave sleep induces brain connectivity changes related to memory stabilization
Abstract
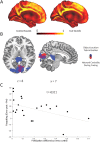
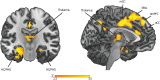
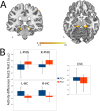
Memory reprocessing following acquisition enhances memory consolidation. Specifically, neural activity during encoding is thought to be 'replayed' during subsequent slow-wave sleep. Such memory replay is thought to contribute to the functional reorganization of neural memory traces. In particular, memory replay may facilitate the exchange of information across brain regions by inducing a reconfiguration of connectivity across the brain. Memory reactivation can be induced by external cues through a procedure known as "targeted memory reactivation". Here, we analysed data from a published study with auditory cues used to reactivate visual object-location memories during slow-wave sleep. We characterized effects of memory reactivation on brain network connectivity using graph-theory. We found that cue presentation during slow-wave sleep increased global network integration of occipital cortex, a visual region that was also active during retrieval of object locations. Although cueing did not have an overall beneficial effect on the retention of cued versus uncued associations, individual differences in overnight memory stabilization were related to enhanced network integration of occipital cortex. Furthermore, occipital cortex displayed enhanced connectivity with mnemonic regions, namely the hippocampus, parahippocampal gyrus, thalamus and medial prefrontal cortex during cue sound presentation. Together, these results suggest a neural mechanism where cue-induced replay during sleep increases integration of task-relevant perceptual regions with mnemonic regions. This cross-regional integration may be instrumental for the consolidation and long-term storage of enduring memories.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

