Endothelial cell-specific anticoagulation reduces inflammation in a mouse model of acute lung injury
- PMID: 30446733
- PMCID: PMC6786365
- DOI: 10.1038/s41401-018-0175-7
Endothelial cell-specific anticoagulation reduces inflammation in a mouse model of acute lung injury
Abstract
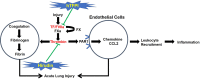
Tissue factor (TF)-dependent coagulation contributes to lung inflammation and the pathogenesis of acute lung injury (ALI). In this study, we explored the roles of targeted endothelial anticoagulation in ALI using two strains of transgenic mice expressing either a membrane-tethered human tissue factor pathway inhibitor (hTFPI) or hirudin fusion protein on CD31+ cells, including vascular endothelial cells (ECs). ALI was induced by intratracheal injection of LPS, and after 24 h the expression of TF and protease-activated receptors (PARs) on EC in lungs were assessed, alongside the extent of inflammation and injury. The expression of TF and PARs on the EC in lungs was upregulated after ALI. In the two strains of transgenic mice, expression of either of hTFPI or hirudin by EC was associated with significant reduction of inflammation, as assessed by the extent of leukocyte infiltration or the levels of proinflammatory cytokines, and promoted survival after LPS-induced ALI. The beneficial outcomes were associated with inhibition of the expression of chemokine CCL2 in lung tissues. The protection observed in the CD31-TFPI-transgenic strain was abolished by injection of an anti-hTFPI antibody, but not by prior engraftment of the transgenic strains with WT bone marrow, confirming that the changes observed were a specific transgenic expression of anticoagulants by EC. These results demonstrate that the inflammation in ALI is TF and thrombin dependent, and that expression of anticoagulants by EC significantly inhibits the development of ALI via repression of leukocyte infiltration, most likely via inhibition of chemokine gradients. These data enhance our understanding of the pathology of ALI and suggest a novel therapeutic strategy for treatment.
Keywords: CCL2; acute lung injury; anticoagulants; endothelial cells; hirudin; human tissue factor pathway inhibitor (hTFPI); inflammation; lipopolysaccharide.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- van Deventer SJ, Buller HR, ten Cate JW, Aarden LA, Hack CE, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–6. - PubMed
-
- Idell S, Koenig KB, Fair DS, Martin TR, McLarty J, Maunder RJ. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;261:L240–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

