Real-World Use and Outcomes of Olaparib: a Population-Based Cohort Study
- PMID: 30446872
- PMCID: PMC6297279
- DOI: 10.1007/s11523-018-0604-z
Real-World Use and Outcomes of Olaparib: a Population-Based Cohort Study
Abstract
Background: Although olaparib, the first poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor approved, has been used in routine clinical practice for over three years, little has been published on its uptake, utilization patterns, and clinical outcomes.
Objective: To examine real-world use and outcomes of olaparib treatment in Swedish patients during the first three years following regulatory approval.
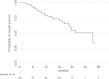
Patients and methods: This is a population-based cohort study using data from the Swedish national registers. All individuals initiating olaparib treatment from regulatory approval to 31 December 2017 were included. The extent of off-label use was assessed based on recorded diagnoses. Ovarian cancer patients were followed until death or the end of the study period. Starting dose and dose adjustments were assessed. Time to olaparib discontinuation and overall survival were plotted using Kaplan-Meier survival curves.
Results: We identified 109 patients to whom olaparib was dispensed in Sweden during the study period. Nine of these were prescribed olaparib off-label for either breast or prostate cancer and were excluded from further analyses. Median age among the remaining 100 patients with ovarian cancer was 59 years (range: 42-83). Almost all patients (96%) started on the recommended dose (400 mg [eight capsules] taken twice daily). Dose reductions were explicitly recorded for 14% of patients. Median time to discontinuation was 289 days (95% confidence interval [CI]: 226; 338). Median overall survival from olaparib initiation was 1002 days (95% CI: 676; not calculable).
Conclusions: To our knowledge, this is the first population-based study of olaparib real-world use and outcomes. During the first three years following regulatory approval, olaparib was mainly prescribed to ovarian cancer patients. Ovarian cancer patients stayed on olaparib for a median of 9.5 months and the treatment appeared to be well tolerated.
Conflict of interest statement
IE, BW, and KB declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Figures
References
-
- IQVIA Institute for Human Data Science. Global Oncology Trends 2018: Innovation, Expansion and Disruption. 2018.
-
- Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/s1470-2045(14)70228-1. - DOI - PubMed
-
- Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/s1470-2045(17)30469-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources