SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018)
- PMID: 30446985
- PMCID: PMC6339680
- DOI: 10.1007/s12094-018-1978-1
SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018)
Abstract
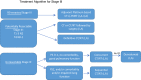
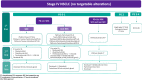
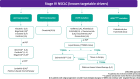
Non-small cell lung cancer (NSCLC) accounts for up to 85% of all lung cancers. The last few years have seen the development of a new staging system, diagnostic procedures such as liquid biopsy, treatments like immunotherapy, as well as deeper molecular knowledge; so, more options can be offered to patients with driver mutations. Groups with specific treatments account for around 25% and demonstrate significant increases in overall survival, and in some subgroups, it is important to evaluate each treatment alternative in accordance with scientific evidence, and even more so with immunotherapy. New treatments similarly mean that we must reconsider what should be done in oligometastatic disease where local treatment attains greater value.
Keywords: Chemotherapy; Immunotherapy; NSCLC; Radiotherapy; Targeted therapies.
Conflict of interest statement
Conflict of interest
MM reports personal fees from AstraZeneca, Roche, Lilly, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Novartis, Tesaro, outside the submitted work. OJ reports grants from Astra Zeneca and personal fees from Astra Zeneca, BMS, MSD, ROCHE, Boehringer Ingelheim, Lilly outside the submitted work. MP reports grants from BMS, Roche and personal fees from BMS, MSD, ROCHE, Pierre Fabre, Novartis; Takeda, Lilly, Pfizer outside the submitted work. NR reports advisory Board/Consultancy/Speaker honoraria from MSD, Bristol-Myers, Roche, Boehringer Ingelheim, Guardant Health, Pfizer, Abbie, Ipsen, Novartis, Astra-Zeneca, Lilly, Takeda outside the submitted work; Research Funding from Pfizer, NanoString Technology, and Institutional financial interests related to clinical trials and patient recruitment. GC reports non-financial support from Millennium Pharmaceuticals, Inc., during the conduct of the study; personal fees from ARIAD, AstraZeneca, Roche, Pfizer, BMS, Boehringer Ingelheim, MSD outside the submitted work. YG reports personal fees from Roche, Boehriger-ingelheim, Lilly, MSD, Pfizer, BMS outside the submitted work. JMT reports personal fees from Roche, Boehriger-ingelheim, BMS, Astra Zeneca, MerckSerono outside the submitted work. AI, EC, MG, have not reported any potential conflicts of interest.
Ethical approval
The current study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
No informed consent was necessary for this guideline.
Figures
References
-
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. - DOI - PubMed
-
- Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. J Mol Diagn. 2018;20(2):129–159. doi: 10.1016/j.jmoldx.2017.11.004. - DOI - PubMed
-
- Felip E, Concha A, de Castro J, Gomez-Roman J, Garrido P, Ramirez J, et al. Biomarker testing in advanced non-small-cell lung cancer: a national consensus of the spanish society of pathology and the spanish society of medical oncology. Clin Transl Oncol. 2015;17(2):103–112. doi: 10.1007/s12094-014-1248-9. - DOI - PubMed
-
- Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathozlogists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36(9):911–919. doi: 10.1200/JCO.2017.76.7293. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical