Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update
- PMID: 30447069
- PMCID: PMC6576267
- DOI: 10.1002/cpt.1304
Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update
Abstract
Thiopurine methyltransferase (TPMT) activity exhibits a monogenic codominant inheritance and catabolizes thiopurines. TPMT variant alleles are associated with low enzyme activity and pronounced pharmacologic effects of thiopurines. Loss-of-function alleles in the NUDT15 gene are common in Asians and Hispanics and reduce the degradation of active thiopurine nucleotide metabolites, also predisposing to myelosuppression. We provide recommendations for adjusting starting doses of azathioprine, mercaptopurine, and thioguanine based on TPMT and NUDT15 genotypes (updates on www.cpicpgx.org).
© 2018 The Authors Clinical Pharmacology & Therapeutics © 2018 American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declared no competing interests for this work.
Figures
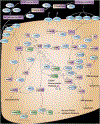
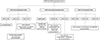
Comment in
-
NUDT15 Polymorphism in Native American Populations of Brazil.Clin Pharmacol Ther. 2019 Jun;105(6):1321-1322. doi: 10.1002/cpt.1379. Epub 2019 Mar 21. Clin Pharmacol Ther. 2019. PMID: 30897207 No abstract available.
References
-
- Sandborn WJ Pharmacogenomics and IBD: TPMT and thiopurines. Inflamm Bowel Dis 10 Suppl 1, S35–7 (2004). - PubMed
-
- Evans WE Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy. Ther Drug Monit 26, 186–91 (2004). - PubMed
-
- Weinshilboum R Inheritance and drug response. N Engl J Med 348, 529–37 (2003). - PubMed
-
- Ford LT & Berg JD Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol 63, 288–95 (2010). - PubMed
-
- Schaeffeler E et al. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants. Pharmacogenetics 14, 407–17 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

