Assessment of Multi-Ion Channel Block in a Phase I Randomized Study Design: Results of the CiPA Phase I ECG Biomarker Validation Study
- PMID: 30447156
- PMCID: PMC6654598
- DOI: 10.1002/cpt.1303
Assessment of Multi-Ion Channel Block in a Phase I Randomized Study Design: Results of the CiPA Phase I ECG Biomarker Validation Study
Abstract
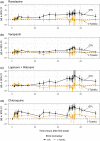
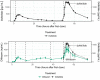
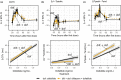
Balanced multi-ion channel-blocking drugs have low torsade risk because they block inward currents. The Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative proposes to use an in silico cardiomyocyte model to determine the presence of balanced block, and absence of heart rate corrected J-Tpeak (J-Tpeak c) prolongation would be expected for balanced blockers. This study included three balanced blockers in a 10-subject-per-drug parallel design; lopinavir/ritonavir and verapamil met the primary end point of ΔΔJ-Tpeak c upper bound < 10 ms, whereas ranolazine did not (upper bounds of 8.8, 6.1, and 12.0 ms, respectively). Chloroquine, a predominant blocker of the potassium channel encoded by the ether-à-go-go related gene (hERG), prolonged ΔΔQTc and ΔΔJ-Tpeak c by ≥ 10 ms. In a separate crossover design, diltiazem (calcium block) did not shorten dofetilide-induced ΔQTc prolongation, but shortened ΔJ-Tpeak c and prolonged ΔTpeak -Tend . Absence of J-Tpeak c prolongation seems consistent with balanced block; however, small sample size (10 subjects) may be insufficient to characterize concentration-response in some cases.
Trial registration: ClinicalTrials.gov NCT03070470.
Published 2019. This article is a U.S. Government work and is in the public domain in the USA. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
P.T.S. has consulting agreements with Biomedical Systems, Charles River, and iCardiac. The other authors report no conflicts of interest.
Figures



References
-
- International Council on Harmonisation . ICH topic S7B the nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals <http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Saf...> (2005). - PubMed
-
- International Council on Harmonisation . Guideline for industry E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs <https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Ef...> (2005). - PubMed
-
- January, C.T. & Riddle, J.M. Early afterdepolarizations: mechanism of induction and block. A role for L‐type Ca2+ current. Circ. Res. 64, 977–990 (1989). - PubMed
-
- Duff, H.J. , Roden, D. , Primm, R.K. , Oates, J.A. & Woosley, R.L. Mexiletine in the treatment of resistant ventricular arrhythmias: enhancement of efficacy and reduction of dose‐related side effects by combination with quinidine. Circulation 67, 1124–1128 (1983). - PubMed
-
- Duff, H. , Mitchell, B. , Manyari, D. & Wyse, G. Mexiletine‐quinidine combination: electrophysiologic correlates of a favorable antiarrhythmic interaction in humans. J. Am. Coll. Cardiol. 10, 1149–1156 (1987). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

