How the visual brain detects emotional changes in facial expressions: Evidence from driven and intrinsic brain oscillations
- PMID: 30447483
- PMCID: PMC6358503
- DOI: 10.1016/j.cortex.2018.10.006
How the visual brain detects emotional changes in facial expressions: Evidence from driven and intrinsic brain oscillations
Abstract
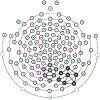
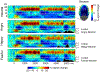
The processing of facial expressions is often studied using static pictorial cues. Recent work, however, suggests that viewing changing expressions more robustly evokes physiological responses. Here, we examined the sensitivity of steady-state visual evoked potentials and intrinsic oscillatory brain activity to transient emotional changes in facial expressions. Twenty-two participants viewed sequences of grayscale faces periodically turned on and off at a rate of 17.5 Hz, to evoke flicker steady-state visual evoked potentials (ssVEPs) in visual cortex. Each sequence began with a neutral face (flickering for 2290 msec), immediately followed by a face from the same actor (also flickering for 2290 msec) with one of four expressions (happy, angry, fearful, or another neutral expression), followed by the initially presented neutral face (flickering for 1140 msec). The amplitude of the ssVEP and the power of intrinsic brain oscillations were analyzed, comparing the four expression-change conditions. We found a transient perturbation (reduction) of the ssVEP that was more pronounced after the neutral-to-angry change compared to the other conditions, at right posterior sensors. Induced alpha-band (8-13 Hz) power was reduced compared to baseline after each change. This reduction showed a central-occipital topography and was strongest in the subtlest and rarest neutral-to-neutral condition. Thus, the ssVEP indexed involvement of face-sensitive cortical areas in decoding affective expressions, whereas mid-occipital alpha power reduction reflected condition frequency rather than expression-specific processing, consistent with the role of alpha power changes in selective attention.
Keywords: Alpha-band oscillations; EEG; Face processing; Facial expressions; ssVEP.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest: none.
Figures
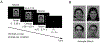




Similar articles
-
Social vision: sustained perceptual enhancement of affective facial cues in social anxiety.Neuroimage. 2011 Jan 15;54(2):1615-24. doi: 10.1016/j.neuroimage.2010.08.080. Epub 2010 Sep 9. Neuroimage. 2011. PMID: 20832490 Free PMC article.
-
Attentional bias to affective faces and complex IAPS images in early visual cortex follows emotional cue extraction.Neuroimage. 2015 May 15;112:254-266. doi: 10.1016/j.neuroimage.2015.03.052. Epub 2015 Mar 25. Neuroimage. 2015. PMID: 25818682
-
Fearful faces heighten the cortical representation of contextual threat.Neuroimage. 2014 Feb 1;86:317-25. doi: 10.1016/j.neuroimage.2013.10.008. Epub 2013 Oct 12. Neuroimage. 2014. PMID: 24125792 Free PMC article.
-
Steady-state visual evoked potentials as a research tool in social affective neuroscience.Psychophysiology. 2016 Dec;53(12):1763-1775. doi: 10.1111/psyp.12768. Epub 2016 Oct 4. Psychophysiology. 2016. PMID: 27699794 Free PMC article. Review.
-
Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging.Neuropsychologia. 2007 Jan 7;45(1):174-94. doi: 10.1016/j.neuropsychologia.2006.06.003. Epub 2006 Jul 18. Neuropsychologia. 2007. PMID: 16854439 Review.
Cited by
-
Social aversive generalization learning sharpens the tuning of visuocortical neurons to facial identity cues.Elife. 2020 Jun 9;9:e55204. doi: 10.7554/eLife.55204. Elife. 2020. PMID: 32515731 Free PMC article.
-
Oscillatory brain dynamics underlying affective face processing.Soc Cogn Affect Neurosci. 2025 May 21;20(1):nsaf047. doi: 10.1093/scan/nsaf047. Soc Cogn Affect Neurosci. 2025. PMID: 40324903 Free PMC article.
-
Interactive effects of social media use and puberty on resting-state cortical activity and mental health symptoms.Dev Cogn Neurosci. 2025 Jan;71:101479. doi: 10.1016/j.dcn.2024.101479. Epub 2024 Nov 22. Dev Cogn Neurosci. 2025. PMID: 39608108 Free PMC article.
References
-
- Aftanas LI, Reva NV, Varlamov AA, Pavlov SV, & Makhnev VP (2004). Analysis of evoked EEG synchronization and desynchronization in conditions of emotional activation in humans: Temporal and topographic characteristics. Neuroscience and Behavioral Physiology, 34(8), 859–867. 10.1023/B:NEAB.0000038139.39812.eb - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

