Different Fgfs have distinct roles in regulating neurogenesis after spinal cord injury in zebrafish
- PMID: 30447699
- PMCID: PMC6240426
- DOI: 10.1186/s13064-018-0122-9
Different Fgfs have distinct roles in regulating neurogenesis after spinal cord injury in zebrafish
Abstract
Background: Despite conserved developmental processes and organization of the vertebrate central nervous system, only some vertebrates including zebrafish can efficiently regenerate neural damage including after spinal cord injury. The mammalian spinal cord shows very limited regeneration and neurogenesis, resulting in permanent life-long functional impairment. Therefore, there is an urgent need to identify the cellular and molecular mechanisms that can drive efficient vertebrate neurogenesis following injury. A key pathway implicated in zebrafish neurogenesis is fibroblast growth factor signaling.
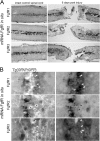
Methods: In the present study we investigated the roles of distinct fibroblast growth factor members and their receptors in facilitating different aspects of neural development and regeneration at different timepoints following spinal cord injury. After spinal cord injury in adults and during larval development, loss and/or gain of Fgf signaling was combined with immunohistochemistry, in situ hybridization and transgenes marking motor neuron populations in in vivo zebrafish and in vitro mammalian PC12 cell culture models.
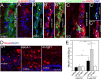
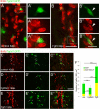
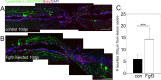
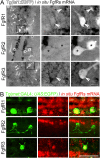
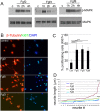
Results: Fgf3 drives neurogenesis of Islet1 expressing motor neuron subtypes and mediate axonogenesis in cMet expressing motor neuron subtypes. We also demonstrate that the role of Fgf members are not necessarily simple recapitulating development. During development Fgf2, Fgf3 and Fgf8 mediate neurogenesis of Islet1 expressing neurons and neuronal sprouting of both, Islet1 and cMet expressing motor neurons. Strikingly in mammalian PC12 cells, all three Fgfs increased cell proliferation, however, only Fgf2 and to some extent Fgf8, but not Fgf3 facilitated neurite outgrowth.
Conclusions: This study demonstrates differential Fgf member roles during neural development and adult regeneration, including in driving neural proliferation and neurite outgrowth of distinct spinal cord neuron populations, suggesting that factors including Fgf type, age of the organism, timing of expression, requirements for different neuronal populations could be tailored to best drive all of the required regenerative processes.
Keywords: C-met; Fgf2; Fgf3; Fgf8; Islet 1; Motor neuron; Neural regeneration.
Conflict of interest statement
Ethics approval and consent to participate
All experiments were conducted in accordance with Monash University guidelines and approved by the local ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Dono R. Fibroblast growth factors as regulators of central nervous system development and function. Am J Phys Regul Integr Comp Phys. 2003;284(4):R867–R881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

