Anti-inflammatory effects induced by pharmaceutical substances on inflammatory active brain astrocytes-promising treatment of neuroinflammation
- PMID: 30447700
- PMCID: PMC6240424
- DOI: 10.1186/s12974-018-1361-8
Anti-inflammatory effects induced by pharmaceutical substances on inflammatory active brain astrocytes-promising treatment of neuroinflammation
Abstract
Background: Pharmaceutical treatment with probable anti-inflammatory substances that attack cells in various ways including receptors, ion channels, or transporter systems may slow down the progression of inflammatory conditions. Astrocytes and microglia are the most prominent target cells for inflammation in the central nervous system. Their responses upon inflammatory stimuli work through the NO/cyclic GMP/protein kinase G systems that can downregulate the ATP-induced Ca2+ signaling, as well as G protein activities which alter Na+ transporters including Na+/K+-ATPase pump activity, Toll-like receptor 4 (TLR4), glutamate-induced Ca2+ signaling, and release of pro-inflammatory cytokines. The rationale for this project was to investigate a combination of pharmaceutical substances influencing the NO and the Gi/Gs activations of inflammatory reactive cells in order to make the cells return into a more physiological state. The ATP-evoked Ca2+ signaling is important maybe due to increased ATP release and subsequent activation of purinergic receptors. A balance between intercellular Ca2+ signaling through gap junctions and extracellular signaling mediated by extracellular ATP may be important for physiological function.
Methods: Astrocytes in primary cultures were incubated with lipopolysaccharide in a physiological glucose concentration for 24 h to induce inflammatory reactivity. The probable anti-inflammatory substances sildenafil and 1α,25-Dihydroxyvitamin D3 together with endomorphin-1, naloxone, and levetiracetam, were used in the presence of high glucose concentration in the medium to restore the cells. Glutamate-, 5-HT-, and ATP-evoked intracellular Ca2+ release, Na+/K+-ATPase expression, expression of inflammatory receptors, and release of tumor necrosis factor alpha were measured.
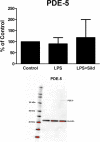
Results: Sildenafil in ultralow concentration together with 1α,25-Dihydroxyvitamin D3 showed most prominent effects on the ATP-evoked intracellular Ca2+ release. The μ-opioid agonist endomorphin-1, the μ-opioid antagonist naloxone in ultralow concentration, and the antiepileptic agent levetiracetam downregulated the glutamate-evoked intracellular Ca2+ release and TLR4. The combination of the pharmaceutical substances in high glucose concentration downregulated the glutamate- and ATP-evoked Ca2+ signaling and the TLR4 expression and upregulated the Na+/K+-ATPase pump.
Conclusion: Pharmaceutical treatment with the combination of substances that have potential anti-inflammatory effects, which attack different biochemical mechanisms in the cells may exert decisive effects to downregulate neuroinflammation in the nervous system.
Keywords: Astrocytes; Ca2+ signaling; Gap junction-coupled cells; Glucose; Inflammation; Na+/K+-ATPase; Pharmaceuticals; Restoration; TLR4.
Conflict of interest statement
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
We have bought the cell cultures from 3H Biomedical Science, Uppsala, Sweden, and used the cultures according to the manufacturer’s instructions. Therefore, no ethics approval can be done.
Consent for publication
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Rönnbäck C, Hansson E. Gap junction coupled cells, barriers and systemic inflammation. Int J Open Access Ophthalmol. 2017;2(1):7. doi: 10.15226/2474-9249/2/1/00117. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

