Older Blood Is Associated With Increased Mortality and Adverse Events in Massively Transfused Trauma Patients: Secondary Analysis of the PROPPR Trial
- PMID: 30447946
- PMCID: PMC6517091
- DOI: 10.1016/j.annemergmed.2018.09.033
Older Blood Is Associated With Increased Mortality and Adverse Events in Massively Transfused Trauma Patients: Secondary Analysis of the PROPPR Trial
Abstract
Study objective: The transfusion of older packed RBCs may be harmful in critically ill patients. We seek to determine the association between packed RBC age and mortality among trauma patients requiring massive packed RBC transfusion.
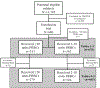
Methods: We analyzed data from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios trial. Subjects in the parent trial included critically injured adult patients admitted to 1 of 12 North American Level I trauma centers who received at least 1 unit of packed RBCs and were predicted to require massive blood transfusion. The primary exposure was volume of packed RBC units transfused during the first 24 hours of hospitalization, stratified by packed RBC age category: 0 to 7 days, 8 to 14 days, 15 to 21 days, and greater than or equal to 22 days. The primary outcome was 24-hour mortality. We evaluated the association between transfused volume of each packed RBC age category and 24-hour survival, using random-effects logistic regression, adjusting for total packed RBC volume, patient age, sex, race, mechanism of injury, Injury Severity Score, Revised Trauma Score, clinical site, and trial treatment group.
Results: The 678 patients included in the analysis received a total of 8,830 packed RBC units. One hundred patients (14.8%) died within the first 24 hours. On multivariable analysis, the number of packed RBCs greater than or equal to 22 days old was independently associated with increased 24-hour mortality (adjusted odds ratio [OR] 1.05 per packed RBC unit; 95% confidence interval [CI] 1.01 to 1.08): OR 0.97 for 0 to 7 days old (95% CI 0.88 to 1.08), OR 1.04 for 8 to 14 days old (95% CI 0.99 to 1.09), and OR 1.02 for 15 to 21 days old (95% CI 0.98 to 1.06). Results of sensitivity analyses were similar only among patients who received greater than or equal to 10 packed RBC units.
Conclusion: Increasing quantities of older packed RBCs are associated with increased likelihood of 24-hour mortality in trauma patients receiving massive packed RBC transfusion (≥10 units), but not in those who receive fewer than 10 units.
Trial registration: ClinicalTrials.gov NCT01545232.
Copyright © 2018 American College of Emergency Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests:
JRH receives patent royalties from the United States Army and the University of Maryland for improved red blood cell storage solutions. The rest of the authors declare that they have no competing interests.
Figures

Comment in
-
Old and New: What Blood Is PROPPR in Trauma Resuscitation?Ann Emerg Med. 2019 Jun;73(6):662-664. doi: 10.1016/j.annemergmed.2019.01.041. Epub 2019 Mar 20. Ann Emerg Med. 2019. PMID: 30902451 No abstract available.
References
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006;60:S3–11. - PubMed
-
- Orlov D, Karkouti K. The pathophysiology and consequences of red blood cell storage. Anaesthesia 2015;70 Suppl 1:29–e12. - PubMed
-
- Hess JR. Measures of stored red blood cell quality. Vox Sang 2014;107:1–9. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

