Light-Activated Dynamic Clamp Using iPSC-Derived Cardiomyocytes
- PMID: 30447994
- PMCID: PMC6289097
- DOI: 10.1016/j.bpj.2018.10.018
Light-Activated Dynamic Clamp Using iPSC-Derived Cardiomyocytes
Abstract
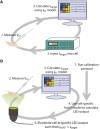
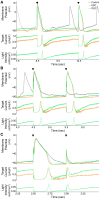
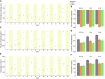
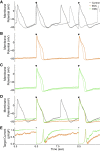
iPSC-derived cardiomyocytes (iPSC-CMs) are a potentially advantageous platform for drug screening because they provide a renewable source of human cardiomyocytes. One obstacle to their implementation is their immature electrophysiology, which reduces relevance to adult arrhythmogenesis. To address this, dynamic clamp is used to inject current representing the insufficient potassium current, IK1, thereby producing more adult-like electrophysiology. However, dynamic clamp requires patch clamp and is therefore low throughput and ill-suited for large-scale drug screening. Here, we use optogenetics to generate such a dynamic-clamp current. The optical dynamic clamp (ODC) uses outward-current-generating opsin, ArchT, to mimic IK1, resulting in more adult-like action potential morphology, similar to IK1 injection via classic dynamic clamp. Furthermore, in the presence of an IKr blocker, ODC revealed expected action potential prolongation and reduced spontaneous excitation. The ODC presented here still requires an electrode to measure Vm but provides a first step toward contactless dynamic clamp, which will not only enable high-throughput screening but may also allow control within multicellular iPSC-CM formats to better recapitulate adult in vivo physiology.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Using Light to Endow Stem-Cell-Derived Cardiomyocytes With Virtual IK1 Conductances.Biophys J. 2018 Dec 4;115(11):2079-2080. doi: 10.1016/j.bpj.2018.10.019. Epub 2018 Oct 30. Biophys J. 2018. PMID: 30442328 Free PMC article. No abstract available.
References
-
- Redfern W.S., Carlsson L., Hammond T.G. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003;58:32–45. - PubMed
-
- Stevens J.L., Baker T.K. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov. Today. 2009;14:162–167. - PubMed
-
- Ferri N., Siegl P., Benghozi R. Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacol. Ther. 2013;138:470–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

