Dual Role of the C-Terminal Domain in Osmosensing by Bacterial Osmolyte Transporter ProP
- PMID: 30448037
- PMCID: PMC6289098
- DOI: 10.1016/j.bpj.2018.10.023
Dual Role of the C-Terminal Domain in Osmosensing by Bacterial Osmolyte Transporter ProP
Abstract
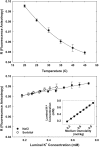
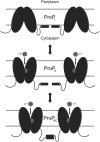
ProP is a member of the major facilitator superfamily, a proton-osmolyte symporter, and an osmosensing transporter. ProP proteins share extended cytoplasmic carboxyl terminal domains (CTDs) implicated in osmosensing. The CTDs of the best characterized, group A ProP orthologs, terminate in sequences that form intermolecular, antiparallel α-helical coiled coils (e.g., ProPEc, from Escherichia coli). Group B orthologs lack that feature (e.g., ProPXc, from Xanthomonas campestris). ProPXc was expressed and characterized in E. coli to further elucidate the role of the coiled coil in osmosensing. The activity of ProPXc was a sigmoid function of the osmolality in cells and proteoliposomes. ProPEc and ProPXc attained similar activities at the same expression level in E. coli. ProPEc transports proline and glycine betaine with comparable high affinities at low osmolality. In contrast, proline weakly inhibited high-affinity glycine-betaine uptake via ProPXc. The KM for proline uptake via ProPEc increases dramatically with the osmolality. The KM for glycine-betaine uptake via ProPXc did not. Thus, ProPXc is an osmosensing transporter, and the C-terminal coiled coil is not essential for osmosensing. The role of CTD-membrane interaction in osmosensing was examined further. As for ProPEc, the ProPXc CTD co-sedimented with liposomes comprising E. coli phospholipid. Molecular dynamics simulations illustrated association of the monomeric ProPEc CTD with the membrane surface. Comparison with the available NMR structure for the homodimeric coiled coil formed by the ProPEc-CTD suggested that membrane association and homodimeric coiled-coil formation by that peptide are mutually exclusive. The membrane fluidity in liposomes comprising E. coli phospholipid decreased with increasing osmolality in the range relevant for ProP activation. These data support the proposal that ProP activates as cellular dehydration increases cytoplasmic cation concentration, releasing the CTD from the membrane surface. For group A orthologs, this also favors α-helical coiled-coil formation that stabilizes the transporter in an active form.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures








Similar articles
-
The osmotic activation of transporter ProP is tuned by both its C-terminal coiled-coil and osmotically induced changes in phospholipid composition.J Biol Chem. 2005 Dec 16;280(50):41387-94. doi: 10.1074/jbc.M508362200. Epub 2005 Oct 20. J Biol Chem. 2005. PMID: 16239220
-
Formation of an antiparallel, intermolecular coiled coil is associated with in vivo dimerization of osmosensor and osmoprotectant transporter ProP in Escherichia coli.Biochemistry. 2005 Aug 2;44(30):10170-80. doi: 10.1021/bi050774y. Biochemistry. 2005. PMID: 16042394
-
The role of the carboxyl terminal alpha-helical coiled-coil domain in osmosensing by transporter ProP of Escherichia coli.J Mol Recognit. 2000 Sep-Oct;13(5):309-22. doi: 10.1002/1099-1352(200009/10)13:5<309::AID-JMR505>3.0.CO;2-R. J Mol Recognit. 2000. PMID: 10992293
-
Osmosensing by bacteria: signals and membrane-based sensors.Microbiol Mol Biol Rev. 1999 Mar;63(1):230-62. doi: 10.1128/MMBR.63.1.230-262.1999. Microbiol Mol Biol Rev. 1999. PMID: 10066837 Free PMC article. Review.
-
BetP of Corynebacterium glutamicum, a transporter with three different functions: betaine transport, osmosensing, and osmoregulation.Biochim Biophys Acta. 2004 Jul 23;1658(1-2):31-6. doi: 10.1016/j.bbabio.2004.05.006. Biochim Biophys Acta. 2004. PMID: 15282171 Review.
Cited by
-
Direct Affinity Purification of Long-Acting PASylated Proteins with Therapeutic Potential Using L-Prolinamide for Mild Elution.Angew Chem Int Ed Engl. 2022 Jun 20;61(25):e202200079. doi: 10.1002/anie.202200079. Epub 2022 Apr 27. Angew Chem Int Ed Engl. 2022. PMID: 35325504 Free PMC article.
-
Exploiting Substrate Promiscuity of Ectoine Hydroxylase for Regio- and Stereoselective Modification of Homoectoine.Front Microbiol. 2019 Nov 27;10:2745. doi: 10.3389/fmicb.2019.02745. eCollection 2019. Front Microbiol. 2019. PMID: 31827466 Free PMC article.
-
Cultivation at high osmotic pressure confers ubiquinone 8-independent protection of respiration on Escherichia coli.J Biol Chem. 2020 Jan 24;295(4):981-993. doi: 10.1074/jbc.RA119.011549. Epub 2019 Dec 11. J Biol Chem. 2020. PMID: 31826918 Free PMC article.
-
Effects of Environmental Stresses on Synthesis of 2-Phenylethanol and IAA by Enterobacter sp. CGMCC 5087.Microorganisms. 2024 Mar 26;12(4):663. doi: 10.3390/microorganisms12040663. Microorganisms. 2024. PMID: 38674607 Free PMC article.
-
Mechanisms underlying the low-temperature adaptation of 17β-estradiol-degrading bacterial strain Rhodococcus sp. RCBS9: insights from physiological and transcriptomic analyses.Front Microbiol. 2024 Nov 21;15:1465627. doi: 10.3389/fmicb.2024.1465627. eCollection 2024. Front Microbiol. 2024. PMID: 39640852 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

