BHLHA15-Positive Secretory Precursor Cells Can Give Rise to Tumors in Intestine and Colon in Mice
- PMID: 30448068
- PMCID: PMC6409180
- DOI: 10.1053/j.gastro.2018.11.024
BHLHA15-Positive Secretory Precursor Cells Can Give Rise to Tumors in Intestine and Colon in Mice
Abstract
Background & aims: The intestinal epithelium is maintained by long-lived intestinal stem cells (ISCs) that reside near the crypt base. Above the ISC zone, there are short-lived progenitors that normally give rise to lineage-specific differentiated cell types but can dedifferentiate into ISCs in certain circumstances. However, the role of epithelial dedifferentiation in cancer development has not been fully elucidated.
Methods: We performed studies with Bhlha15-CreERT, Lgr5-DTR-GFP, Apcflox/flox, LSL-Notch (IC), and R26-reporter strains of mice. Some mice were given diphtheria toxin to ablate Lgr5-positive cells, were irradiated, or were given 5-fluorouracil, hydroxyurea, doxorubicin, or dextran sodium sulfate to induce intestinal or colonic tissue injury. In intestinal tissues, we analyzed the fate of progeny that expressed Bhlha15. We used microarrays and reverse-transcription PCR to analyze gene expression patterns in healthy and injured intestinal tissues and in tumors. We analyzed gene expression patterns in human colorectal tumors using The Cancer Genome Atlas data set.
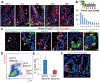
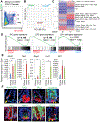
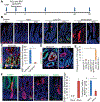
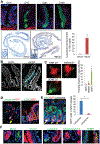
Results: Bhlha15 identified Paneth cells and short-lived secretory precursors (including pre-Paneth label-retaining cells) located just above the ISC zone in the intestinal epithelium. Bhlha15+ cells had no plasticity after loss of Lgr5-positive cells or irradiation. However, Bhlha15+ secretory precursors started to supply the enterocyte lineage after doxorubicin-induced epithelial injury in a Notch-dependent manner. Sustained activation of Notch converts Bhlha15+ secretory precursors to long-lived enterocyte progenitors. Administration of doxorubicin and expression of an activated form of Notch resulted in a gene expression pattern associated with enterocyte progenitors, whereas only sustained activation of Notch altered gene expression patterns in Bhlha15+ precursors toward those of ISCs. Bhlha15+ enterocyte progenitors with sustained activation of Notch formed intestinal tumors with serrated features in mice with disruption of Apc. In the colon, Bhlha15 marked secretory precursors that became stem-like, cancer-initiating cells after dextran sodium sulfate-induced injury, via activation of Src and YAP signaling. In analyses of human colorectal tumors, we associated activation of Notch with chromosome instability-type tumors with serrated features in the left colon.
Conclusions: In mice, we found that short-lived precursors can undergo permanent reprogramming by activation of Notch and YAP signaling. These cells could mediate tumor formation in addition to traditional ISCs.
Keywords: Colon Cancer; Interconversion; Tumorigenesis; Yes Associated Protein 1.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
Disclosures:
The authors disclose no conflicts.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

