Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial
- PMID: 30449623
- PMCID: PMC6319250
- DOI: 10.1016/S0140-6736(18)32752-1
Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial
Abstract
Background: The incidence of human papillomavirus (HPV)-positive oropharyngeal cancer, a disease affecting younger patients, is rapidly increasing. Cetuximab, an epidermal growth factor receptor inhibitor, has been proposed for treatment de-escalation in this setting to reduce the toxicity of standard cisplatin treatment, but no randomised evidence exists for the efficacy of this strategy.
Methods: We did an open-label randomised controlled phase 3 trial at 32 head and neck treatment centres in Ireland, the Netherlands, and the UK, in patients aged 18 years or older with HPV-positive low-risk oropharyngeal cancer (non-smokers or lifetime smokers with a smoking history of <10 pack-years). Eligible patients were randomly assigned (1:1) to receive, in addition to radiotherapy (70 Gy in 35 fractions), either intravenous cisplatin (100 mg/m2 on days 1, 22, and 43 of radiotherapy) or intravenous cetuximab (400 mg/m2 loading dose followed by seven weekly infusions of 250 mg/m2). The primary outcome was overall severe (grade 3-5) toxicity events at 24 months from the end of treatment. The primary outcome was assessed by intention-to-treat and per-protocol analyses. This trial is registered with the ISRCTN registry, number ISRCTN33522080.
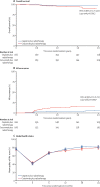
Findings: Between Nov 12, 2012, and Oct 1, 2016, 334 patients were recruited (166 in the cisplatin group and 168 in the cetuximab group). Overall (acute and late) severe (grade 3-5) toxicity did not differ significantly between treatment groups at 24 months (mean number of events per patient 4·8 [95% CI 4·2-5·4] with cisplatin vs 4·8 [4·2-5·4] with cetuximab; p=0·98). At 24 months, overall all-grade toxicity did not differ significantly either (mean number of events per patient 29·2 [95% CI 27·3-31·0] with cisplatin vs 30·1 [28·3-31·9] with cetuximab; p=0·49). However, there was a significant difference between cisplatin and cetuximab in 2-year overall survival (97·5% vs 89·4%, hazard ratio 5·0 [95% CI 1·7-14·7]; p=0·001) and 2-year recurrence (6·0% vs 16·1%, 3·4 [1·6-7·2]; p=0·0007).
Interpretation: Compared with the standard cisplatin regimen, cetuximab showed no benefit in terms of reduced toxicity, but instead showed significant detriment in terms of tumour control. Cisplatin and radiotherapy should be used as the standard of care for HPV-positive low-risk patients who are able to tolerate cisplatin.
Funding: Cancer Research UK.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
De-intensified treatment in human papillomavirus-positive oropharyngeal cancer.Lancet. 2019 Jan 5;393(10166):5-7. doi: 10.1016/S0140-6736(18)32930-1. Epub 2018 Nov 15. Lancet. 2019. PMID: 30449624 No abstract available.
-
[In HPV-associated oropharyngeal cancer, chemoradiotherapy with cisplatin is superior to bioradiotherapy with cetuximab in terms of overall survival].Strahlenther Onkol. 2019 May;195(5):447-449. doi: 10.1007/s00066-019-01441-w. Strahlenther Onkol. 2019. PMID: 30789992 German. No abstract available.
-
De-Escalation Strategies in HPV-Associated Oropharynx Cancer-Are we Putting the Cart Before the Horse?Int J Radiat Oncol Biol Phys. 2019 Jul 15;104(4):705-709. doi: 10.1016/j.ijrobp.2019.02.054. Int J Radiat Oncol Biol Phys. 2019. PMID: 31204653 Free PMC article. No abstract available.
-
[Radiochemotherapy of HPV-positive oropharyngeal carcinomas: Cisplatin is still to be prioritized].Bull Cancer. 2020 Mar;107(3):293-294. doi: 10.1016/j.bulcan.2020.02.002. Epub 2020 Feb 26. Bull Cancer. 2020. PMID: 32113612 French. No abstract available.
References
-
- Castellsague X, Alemany L, Quer M. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108:djv403. - PubMed
-
- Mehanna H, Beech T, Nicholson T. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

