Ectonucleoside Triphosphate Diphosphohydrolase-3 Antibody Targets Adult Human Pancreatic β Cells for In Vitro and In Vivo Analysis
- PMID: 30449685
- PMCID: PMC6402969
- DOI: 10.1016/j.cmet.2018.10.007
Ectonucleoside Triphosphate Diphosphohydrolase-3 Antibody Targets Adult Human Pancreatic β Cells for In Vitro and In Vivo Analysis
Abstract
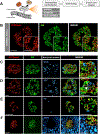
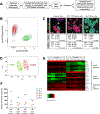
Identification of cell-surface markers specific to human pancreatic β cells would allow in vivo analysis and imaging. Here we introduce a biomarker, ectonucleoside triphosphate diphosphohydrolase-3 (NTPDase3), that is expressed on the cell surface of essentially all adult human β cells, including those from individuals with type 1 or type 2 diabetes. NTPDase3 is expressed dynamically during postnatal human pancreas development, appearing first in acinar cells at birth, but several months later its expression declines in acinar cells while concurrently emerging in islet β cells. Given its specificity and membrane localization, we utilized an NTPDase3 antibody for purification of live human β cells as confirmed by transcriptional profiling, and, in addition, for in vivo imaging of transplanted human β cells. Thus, NTPDase3 is a cell-surface biomarker of adult human β cells, and the antibody directed to this protein should be a useful new reagent for β cell sorting, in vivo imaging, and targeting.
Keywords: development; diabetes; glucagon; imaging; insulin; islet; pancreas.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The NTPDase3 antibody used in the manuscript is available for purchase (
Figures



Similar articles
-
A Map of Human Type 1 Diabetes Progression by Imaging Mass Cytometry.Cell Metab. 2019 Mar 5;29(3):755-768.e5. doi: 10.1016/j.cmet.2018.11.014. Epub 2019 Jan 31. Cell Metab. 2019. PMID: 30713109 Free PMC article.
-
Pancreatic Alpha-Cells Contribute Together With Beta-Cells to CXCL10 Expression in Type 1 Diabetes.Front Endocrinol (Lausanne). 2020 Sep 15;11:630. doi: 10.3389/fendo.2020.00630. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042009 Free PMC article.
-
In Vivo Visualization of β-Cells by Targeting of GPR44.Diabetes. 2018 Feb;67(2):182-192. doi: 10.2337/db17-0764. Epub 2017 Dec 5. Diabetes. 2018. PMID: 29208633
-
Organisation of the human pancreas in health and in diabetes.Diabetologia. 2020 Oct;63(10):1966-1973. doi: 10.1007/s00125-020-05203-7. Epub 2020 Sep 7. Diabetologia. 2020. PMID: 32894306 Free PMC article. Review.
-
Fifty years of pancreatic islet pathology in human type 1 diabetes: insights gained and progress made.Diabetologia. 2018 Dec;61(12):2499-2506. doi: 10.1007/s00125-018-4731-y. Epub 2018 Sep 25. Diabetologia. 2018. PMID: 30255378 Free PMC article. Review.
Cited by
-
The Eye as a Transplantation Site to Monitor Pancreatic Islet Cell Plasticity.Front Endocrinol (Lausanne). 2021 Apr 23;12:652853. doi: 10.3389/fendo.2021.652853. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33967961 Free PMC article. Review.
-
Exploring the Molecular Mechanism of Comorbidity of Type 2 Diabetes Mellitus and Colorectal Cancer: Insights from Bulk Omics and Single-Cell Sequencing Validation.Biomolecules. 2024 Jun 14;14(6):693. doi: 10.3390/biom14060693. Biomolecules. 2024. PMID: 38927096 Free PMC article.
-
Genetic risk converges on regulatory networks mediating early type 2 diabetes.Nature. 2023 Dec;624(7992):621-629. doi: 10.1038/s41586-023-06693-2. Epub 2023 Dec 4. Nature. 2023. PMID: 38049589 Free PMC article.
-
New Insights into Immunotherapy Strategies for Treating Autoimmune Diabetes.Int J Mol Sci. 2019 Sep 26;20(19):4789. doi: 10.3390/ijms20194789. Int J Mol Sci. 2019. PMID: 31561568 Free PMC article. Review.
-
Human Beta Cell Regenerative Drug Therapy for Diabetes: Past Achievements and Future Challenges.Front Endocrinol (Lausanne). 2021 Jul 16;12:671946. doi: 10.3389/fendo.2021.671946. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34335466 Free PMC article. Review.
References
-
- Aamodt KI, Aramandla R, Brown JJ, Fiaschi-Taesch N, Wang P, Stewart AF, Brissova M, and Powers AC (2016). Development of a reliable automated screening system to identify small molecules and biologics that promote human β-cell regeneration. Am. J. Physiol. Endocrinol. Metab 311, E859–E868. doi:10.1152/ajpendo.00515.2015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- UC4 DK104218/DK/NIDDK NIH HHS/United States
- UC4 DK104211/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- UC4 DK112232/DK/NIDDK NIH HHS/United States
- U01 DK089572/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- F30 DK118830/DK/NIDDK NIH HHS/United States
- U42 OD011158/OD/NIH HHS/United States
- UC4 DK098085/DK/NIDDK NIH HHS/United States
- UC4 DK108120/DK/NIDDK NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- U24 DK098085/DK/NIDDK NIH HHS/United States
- U2C DK059637/DK/NIDDK NIH HHS/United States
- I01 BX000666/BX/BLRD VA/United States
- R01 DK094199/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- R24 DK106755/DK/NIDDK NIH HHS/United States
- K01 DK117969/DK/NIDDK NIH HHS/United States
- U01 DK104218/DK/NIDDK NIH HHS/United States
- S10 OD016245/OD/NIH HHS/United States
- R01 DK097829/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- U01 DK123716/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

