Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging
- PMID: 30449736
- PMCID: PMC6286850
- DOI: 10.18632/aging.101643
Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging
Abstract
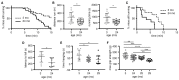
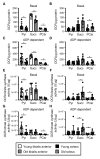
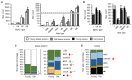
Preservation of mitochondrial function, which is dependent on mitochondrial homeostasis (biogenesis, dynamics, disposal/recycling), is critical for maintenance of skeletal muscle function. Skeletal muscle performance declines upon aging (sarcopenia) and is accompanied by decreased mitochondrial function in fast-glycolytic muscles. Oxidative metabolism promotes mitochondrial homeostasis, so we investigated whether mitochondrial function is preserved in oxidative muscles. We compared tibialis anterior (predominantly glycolytic) and soleus (oxidative) muscles from young (3 mo) and old (28-29 mo) C57BL/6J mice. Throughout life, the soleus remained more oxidative than the tibialis anterior and expressed higher levels of markers of mitochondrial biogenesis, fission/fusion and autophagy. The respiratory capacity of mitochondria isolated from the tibialis anterior, but not the soleus, declined upon aging. The soleus and tibialis anterior exhibited similar aging-associated changes in mitochondrial biogenesis, fission/fusion, disposal and autophagy marker expression, but opposite changes in fiber composition: the most oxidative fibers declined in the tibialis anterior, while the more glycolytic fibers declined in the soleus. In conclusion, oxidative muscles are protected from mitochondrial aging, probably due to better mitochondrial homeostasis ab initio and aging-associated changes in fiber composition. Exercise training aimed at enriching oxidative fibers may be valuable in preventing mitochondria-related aging and its contribution to sarcopenia.
Keywords: aging; glycolytic; mitochondria; oxidative; sarcopenia; skeletal muscle.
Conflict of interest statement
Figures



References
-
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Biran M, Deschodt-Arsac V, Miraux S, Thiaudiere E, Pasdois P, Detaille D, Franconi JM, Babot M, Trézéguet V, et al. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell. 2014; 13:39–48. 10.1111/acel.12147 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

