Pathogen dynamics under both bottom-up host resistance and top-down hyperparasite attack
- PMID: 30449900
- PMCID: PMC6220889
- DOI: 10.1111/1365-2664.13185
Pathogen dynamics under both bottom-up host resistance and top-down hyperparasite attack
Abstract
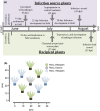
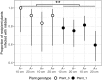
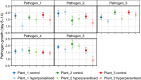
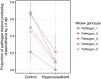
The relative importance of bottom-up versus top-down control of population dynamics has been the focus of much debate. In infectious disease biology, research is typically focused on the bottom-up process of host resistance, wherein the direction of control flows from the lower to the higher trophic level to impact on pathogen population size and epidemiology. However, the importance of top-down control by a pathogen's natural enemies has been mostly overlooked.Here, we explore the effects of, and interaction between, host genotype (i.e., genetic susceptibility to pathogen infection) and infection by a hyperparasitic fungus, Ampelomyces spp., on the establishment and early epidemic growth and transmission of a powdery mildew plant pathogen (Podosphaera plantaginis). We used a semi-natural field experiment to contrast the impacts of hyperparasite infection, host-plant resistance and spatial structure to reveal the key factors that determine pathogen spread. We then used a laboratory-based inoculation approach to test whether the field experiment results hold across multiple pathogen-host genetic combinations and to explore hyperparasite effects on the pathogen's later life-history stages.We found that hyperparasite infection had a negligible effect on within-host infection development and between-host spread of the pathogen during the onset of epidemics. In contrast, host-plant resistance was the major determinant of whether plants became infected, and host genotype and proximity to an infection source determined infection severity.Our laboratory study showed that, while the interaction between host and pathogen genotypes was the key determinant of infection outcome, hyperparasitism did, on average, reduce the severity of infection. Moreover, hyperparasite infection negatively influenced the production of the pathogen's overwintering structures. Synthesis and applications. Our results suggest that bottom-up host resistance affects pathogen spread, but top-down control of powdery mildew pathogens is likely more effective against later life-history stages. Further, while hyperparasitism in this system can reduce early pathogen growth under stable laboratory conditions, this effect is not detectable in a semi-natural environment. Considering the effects of hyperparasites at multiple points in pathogen's life history will be important when considering hyperparasite-derived biocontrol measures in other natural and agricultural systems.
Keywords: Ampelomyces spp.; Plantago lanceolata; Podosphaera plantaginis; bottom‐up; disease biology; hyperparasite; plant pathogen; top‐down.
Figures
References
-
- Abo‐Foul, S. , Raskin, V. I. , Sztejnberg, A. , & Marder, J. B. (1996). Disruption of chlorophyll organization and function in powdery mildew‐diseased cucumber leaves and its control by the hyperparasite Ampelomyces quisqualis . Phytopathology, 86, 195–199. 10.1094/Phyto-86-195 - DOI
-
- Barrera, W. , Hoy, J , & Li, B . (2012). Effects of temperature and moisture variables on brown rust epidemics in sugarcane. Journal of Phytopathology, 161, 98–106.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
