Critical Dependence of Molecular Weight on Thermoresponsive Behavior of Diblock Copolymer Worm Gels in Aqueous Solution
- PMID: 30449901
- PMCID: PMC6236470
- DOI: 10.1021/acs.macromol.8b01617
Critical Dependence of Molecular Weight on Thermoresponsive Behavior of Diblock Copolymer Worm Gels in Aqueous Solution
Abstract
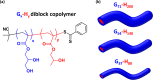
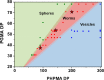
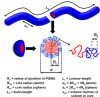
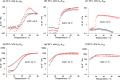
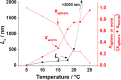
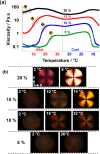
Reversible addition-fragmentation chain transfer (RAFT) aqueous dispersion polymerization of 2-hydroxypropyl methacrylate was used to prepare three poly(glycerol monomethacrylate) x -poly(2-hydroxypropyl methacrylate) y (denoted G x -H y or PGMA-PHPMA) diblock copolymers, namely G37-H80, G54-H140, and G71-H200. A master phase diagram was used to select each copolymer composition to ensure that a pure worm phase was obtained in each case, as confirmed by transmission electron microscopy (TEM) and small-angle x-ray scattering (SAXS) studies. The latter technique indicated a mean worm cross-sectional diameter (or worm width) ranging from 11 to 20 nm as the mean degree of polymerization (DP) of the hydrophobic PHPMA block was increased from 80 to 200. These copolymer worms form soft hydrogels at 20 °C that undergo degelation on cooling. This thermoresponsive behavior was examined using variable temperature DLS, oscillatory rheology, and SAXS. A 10% w/w G37-H80 worm dispersion dissociated to afford an aqueous solution of molecularly dissolved copolymer chains at 2 °C; on returning to ambient temperature, these chains aggregated to form first spheres and then worms, with the original gel strength being recovered. In contrast, the G54-H140 and G71-H200 worms each only formed spheres on cooling to 2 °C, with thermoreversible (de)gelation being observed in the former case. The sphere-to-worm transition for G54-H140 was monitored by variable temperature SAXS: these experiments indicated the gradual formation of longer worms at higher temperature, with a concomitant reduction in the number of spheres, suggesting worm growth via multiple 1D sphere-sphere fusion events. DLS studies indicated that a 0.1% w/w aqueous dispersion of G71-H200 worms underwent an irreversible worm-to-sphere transition on cooling to 2 °C. Furthermore, irreversible degelation over the time scale of the experiment was also observed during rheological studies of a 10% w/w G71-H200 worm dispersion. Shear-induced polarized light imaging (SIPLI) studies revealed qualitatively different thermoreversible behavior for these three copolymer worm dispersions, although worm alignment was observed at a shear rate of 10 s-1 in each case. Subsequently conducting this technique at a lower shear rate of 1 s-1 combined with ultra small-angle x-ray scattering (USAXS) also indicated that worm branching occurred at a certain critical temperature since an upturn in viscosity, distortion in the birefringence, and a characteristic feature in the USAXS pattern were observed. Finally, SIPLI studies indicated that the characteristic relaxation times required for loss of worm alignment after cessation of shear depended markedly on the copolymer molecular weight.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









Similar articles
-
Shear-induced alignment of block copolymer worms in mineral oil.Soft Matter. 2021 Oct 13;17(39):8867-8876. doi: 10.1039/d1sm01011e. Soft Matter. 2021. PMID: 34542137
-
Rational synthesis of novel biocompatible thermoresponsive block copolymer worm gels.Soft Matter. 2021 Jun 9;17(22):5602-5612. doi: 10.1039/d1sm00460c. Soft Matter. 2021. PMID: 33998622
-
Aqueous worm gels can be reconstituted from freeze-dried diblock copolymer powder.Soft Matter. 2014 Jun 14;10(22):3984-92. doi: 10.1039/c4sm00415a. Epub 2014 Apr 14. Soft Matter. 2014. PMID: 24733440
-
Shape-Shifting Thermoresponsive Block Copolymer Nano-Objects.J Colloid Interface Sci. 2023 Mar 15;634:906-920. doi: 10.1016/j.jcis.2022.12.080. Epub 2022 Dec 18. J Colloid Interface Sci. 2023. PMID: 36566636 Review.
-
Recent Advances in Polymerization-Induced Self-Assembly (PISA) Syntheses in Non-Polar Media.Angew Chem Int Ed Engl. 2023 Oct 16;62(42):e202308372. doi: 10.1002/anie.202308372. Epub 2023 Jul 19. Angew Chem Int Ed Engl. 2023. PMID: 37409380 Free PMC article. Review.
Cited by
-
Adaptive Recombinant Nanoworms from Genetically Encodable Star Amphiphiles.Biomacromolecules. 2022 Mar 14;23(3):863-876. doi: 10.1021/acs.biomac.1c01314. Epub 2021 Dec 23. Biomacromolecules. 2022. PMID: 34942072 Free PMC article.
-
Continuous-Flow Laboratory SAXS for In Situ Determination of the Impact of Hydrophilic Block Length on Spherical Nano-Object Formation during Polymerization-Induced Self-Assembly.Macromolecules. 2023 Aug 4;56(16):6426-6435. doi: 10.1021/acs.macromol.3c00585. eCollection 2023 Aug 22. Macromolecules. 2023. PMID: 37637307 Free PMC article.
-
Aldehyde-functional thermoresponsive diblock copolymer worm gels exhibit strong mucoadhesion.Chem Sci. 2022 May 26;13(23):6888-6898. doi: 10.1039/d2sc02074b. eCollection 2022 Jun 15. Chem Sci. 2022. PMID: 35774174 Free PMC article.
-
Efficient Synthesis of Hydrolytically Degradable Block Copolymer Nanoparticles via Reverse Sequence Polymerization-Induced Self-Assembly in Aqueous Media.Angew Chem Int Ed Engl. 2023 Sep 18;62(38):e202309526. doi: 10.1002/anie.202309526. Epub 2023 Aug 10. Angew Chem Int Ed Engl. 2023. PMID: 37522648 Free PMC article.
-
In situ SAXS studies of a prototypical RAFT aqueous dispersion polymerization formulation: monitoring the evolution in copolymer morphology during polymerization-induced self-assembly.Chem Sci. 2020 Sep 18;11(42):11443-11454. doi: 10.1039/d0sc03411h. Chem Sci. 2020. PMID: 34094387 Free PMC article.
References
-
- Bütün V.; Armes S. P.; Billingham N. C. Synthesis and aqueous solution properties of near-monodisperse tertiary amine methacrylate homopolymers and diblock copolymers. Polymer 2001, 42 (14), 5993–6008. 10.1016/S0032-3861(01)00066-0. - DOI
-
- Pearson R. T.; Warren N. J.; Lewis A. L.; Armes S. P.; Battaglia G. Effect of pH and Temperature on PMPC–PDPA Copolymer Self-Assembly. Macromolecules 2013, 46 (4), 1400–1407. 10.1021/ma302228m. - DOI
LinkOut - more resources
Full Text Sources
