Intracellular Follicle-Stimulating Hormone Receptor Trafficking and Signaling
- PMID: 30450081
- PMCID: PMC6225286
- DOI: 10.3389/fendo.2018.00653
Intracellular Follicle-Stimulating Hormone Receptor Trafficking and Signaling
Abstract
Models of G protein-coupled receptor (GPCR) signaling have dramatically altered over the past two decades. Indeed, GPCRs such as the follicle-stimulating hormone receptor (FSHR) have contributed to these new emerging models. We now understand that receptor signaling is highly organized at a spatial level, whereby signaling not only occurs from the plasma membrane but distinct intracellular compartments. Recent studies in the role of membrane trafficking and spatial organization of GPCR signaling in regulating gonadotropin hormone receptor activity has identified novel intracellular compartments, which are tightly linked with receptor signaling and reciprocally regulated by the cellular trafficking machinery. Understanding the impact of these cell biological mechanisms to physiology and pathophysiology is emerging for certain GPCRs. However, for FSHR, the potential impact in both health and disease and the therapeutic possibilities of these newly identified systems is currently unknown, but offers the potential to reassess prior strategies, or unveil novel opportunities, in targeting this receptor.
Keywords: FSH receptor; GPCR; cAMP; endocytosis; endosome; signaling; trafficking.
Figures

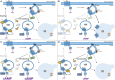
References
-
- Green ED, Baenziger JU. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. (1988) 263:36–44. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

