Improving Immunotherapy Through Glycodesign
- PMID: 30450094
- PMCID: PMC6224361
- DOI: 10.3389/fimmu.2018.02485
Improving Immunotherapy Through Glycodesign
Abstract
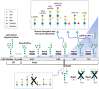
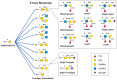
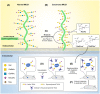
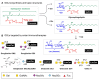
Immunotherapy is revolutionizing health care, with the majority of high impact "drugs" approved in the past decade falling into this category of therapy. Despite considerable success, glycosylation-a key design parameter that ensures safety, optimizes biological response, and influences the pharmacokinetic properties of an immunotherapeutic-has slowed the development of this class of drugs in the past and remains challenging at present. This article describes how optimizing glycosylation through a variety of glycoengineering strategies provides enticing opportunities to not only avoid past pitfalls, but also to substantially improve immunotherapies including antibodies and recombinant proteins, and cell-based therapies. We cover design principles important for early stage pre-clinical development and also discuss how various glycoengineering strategies can augment the biomanufacturing process to ensure the overall effectiveness of immunotherapeutics.
Keywords: antibody-dependent cell cytotoxicity (ADCC); antibody-drug conjugates (ADCs); glycoengineering; glycosylation; immunotherapy; metabolic glycoengineering; monoclonal antibodies.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

