A Strong Humoral Immune Response Induced by a Vaccine Formulation Containing rSm29 Adsorbed to Alum Is Associated With Protection Against Schistosoma mansoni Reinfection in Mice
- PMID: 30450095
- PMCID: PMC6224358
- DOI: 10.3389/fimmu.2018.02488
A Strong Humoral Immune Response Induced by a Vaccine Formulation Containing rSm29 Adsorbed to Alum Is Associated With Protection Against Schistosoma mansoni Reinfection in Mice
Abstract
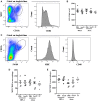
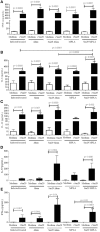
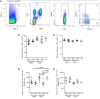
The helminth Schistosoma mansoni is one of main causes of human schistosomiasis, a health and economic concern in some of the world's poorest countries. Current treatment regimens can lead to serious side effects and are not suitable for breastfeeding mothers. As such, efforts have been undertaken to develop a vaccine to prevent infection. Of these, Sm29 is a promising candidate that has been associated with resistance to infection/reinfection in humans and mice. Its ability to induce resistance to reinfection has also been recently demonstrated using a vaccine formulation containing Freund's adjuvant. However, Freund's adjuvant is unsuitable for use in human vaccines. We therefore evaluated the ability of Sm29 to induce protection against S. mansoni reinfection when formulated with either alum or MPLA as an adjuvant, both approved for human use. Our data demonstrate that, in contrast to Sm29 with MPLA, Sm29 with alum reduced parasite burden after reinfection compared to a control. We next investigated whether the immune response was involved in creating the differences between the protective (Sm29Alum) and non-protective (Sm29MPLA) vaccine formulations. We observed that both formulations induced a similar mixed-profile immune response, however, the Sm29 with alum formulation raised the levels of antibodies against Sm29. This suggests that there is an association between a reduction in worm burden and parasite-specific antibodies. In summary, our data show that Sm29 with an alum adjuvant can successfully protect against S. mansoni reinfection in mice, indicating a potentially effective vaccine formulation that could be applied in humans.
Keywords: MPLA-SM; Schistosoma mansoni; Sm29; alum; reinfection; vaccine.
Figures



References
-
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (2015) 386:743–800. 10.1016/S0140-6736(15)60692-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

