Interplay Between the Unfolded Protein Response and Immune Function in the Development of Neurodegenerative Diseases
- PMID: 30450103
- PMCID: PMC6224445
- DOI: 10.3389/fimmu.2018.02541
Interplay Between the Unfolded Protein Response and Immune Function in the Development of Neurodegenerative Diseases
Abstract
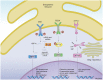
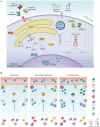
Emerging evidence suggests that the immune and nervous systems are in close interaction in health and disease conditions. Protein aggregation and proteostasis dysfunction at the level of the endoplasmic reticulum (ER) are central contributors to neurodegenerative diseases. The unfolded protein response (UPR) is the main transduction pathway that maintains protein homeostasis under conditions of protein misfolding and aggregation. Brain inflammation often coexists with the degenerative process in different brain diseases. Interestingly, besides its well-described role in neuronal fitness, the UPR has also emerged as a key regulator of ontogeny and function of several immune cell types. Nevertheless, the contribution of the UPR to brain inflammation initiated by immune cells remains largely unexplored. In this review, we provide a perspective on the potential role of ER stress signaling in brain-associated immune cells and the possible implications to neuroinflammation and development of neurodegenerative diseases.
Keywords: ER stress; UPR; immune cells; immune system; inflammation; misfolded proteins; neurodegeneration; protein protein misfolding diseases.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

