Dynamics of the excited-state hydrogen transfer in a (dG)·(dC) homopolymer: intrinsic photostability of DNA
- PMID: 30450180
- PMCID: PMC6202918
- DOI: 10.1039/c8sc03252a
Dynamics of the excited-state hydrogen transfer in a (dG)·(dC) homopolymer: intrinsic photostability of DNA
Abstract
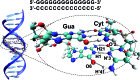
The intrinsic photostability of nucleic acids is intimately related to evolution of life, while its understanding at the molecular and electronic levels remains a challenge for modern science. Among the different decay pathways proposed in the last two decades, the excited-state hydrogen transfer between guanine-cytosine base pairs has been identified as an efficient non-reactive channel to dissipate the excess of energy provided by light absorption. The present work studies the dynamics of such phenomena taking place in a (dG)·(dC) B-DNA homopolymer in water solution using state-of-the-art molecular modelling and simulation methods. A dynamic effect that boosts the photostability of the inter-strand hydrogen atom transfers, inherent to the Watson-Crick base pairing, is unveiled and ascribed to the energy released during the proton transfer step. Our results also reveal a novel mechanism of DNA decay named four proton transfer (FPT), in which two protons of two adjacent G-C base pairs are transferred to form a biradical zwitterionic intermediate. Decay of the latter intermediate to the ground state triggers the transfer of the protons back to the guanine molecules recovering the Watson-Crick structure of the tetramer. This FPT process is activated by the close interaction of a nearby Na+ counterion with the oxygen atoms of the guanine nucleobases and hence represents a photostable channel operative in natural nucleic acids.
Figures





Similar articles
-
Ultraviolet Absorption Induces Hydrogen-Atom Transfer in G⋅C Watson-Crick DNA Base Pairs in Solution.Angew Chem Int Ed Engl. 2015 Dec 1;54(49):14719-22. doi: 10.1002/anie.201506940. Epub 2015 Oct 13. Angew Chem Int Ed Engl. 2015. PMID: 26459502
-
Tautomeric selectivity of the excited-state lifetime of guanine/cytosine base pairs: the role of electron-driven proton-transfer processes.Proc Natl Acad Sci U S A. 2005 Dec 13;102(50):17903-6. doi: 10.1073/pnas.0504087102. Epub 2005 Dec 5. Proc Natl Acad Sci U S A. 2005. PMID: 16330778 Free PMC article.
-
Evidence for Hoogsteen GC base pairs in the proton-induced transition from right-handed to left-handed poly(dG-dC).poly(dG-dC).Biochemistry. 1997 Oct 28;36(43):13241-7. doi: 10.1021/bi971326w. Biochemistry. 1997. PMID: 9341213
-
Excited states in DNA strands investigated by ultrafast laser spectroscopy.Top Curr Chem. 2015;356:39-87. doi: 10.1007/128_2014_570. Top Curr Chem. 2015. PMID: 25326834 Review.
-
Watson-Crick versus Hoogsteen Base Pairs: Chemical Strategy to Encode and Express Genetic Information in Life.Acc Chem Res. 2021 May 4;54(9):2110-2120. doi: 10.1021/acs.accounts.0c00734. Epub 2021 Feb 16. Acc Chem Res. 2021. PMID: 33591181 Review.
Cited by
-
Os(II) Oligothienyl Complexes as a Hypoxia-Active Photosensitizer Class for Photodynamic Therapy.Inorg Chem. 2020 Nov 16;59(22):16341-16360. doi: 10.1021/acs.inorgchem.0c02137. Epub 2020 Oct 30. Inorg Chem. 2020. PMID: 33126792 Free PMC article.
-
The photoactivated dynamics of dGpdC and dCpdG sequences in DNA: a comprehensive quantum mechanical study.Chem Sci. 2024 May 16;15(25):9676-9693. doi: 10.1039/d4sc00910j. eCollection 2024 Jun 26. Chem Sci. 2024. PMID: 38939156 Free PMC article.
-
Spatial and Temporal Resolution of the Oxygen-Independent Photoinduced DNA Interstrand Cross-Linking by a Nitroimidazole Derivative.J Chem Inf Model. 2022 Jul 11;62(13):3239-3252. doi: 10.1021/acs.jcim.2c00460. Epub 2022 Jun 30. J Chem Inf Model. 2022. PMID: 35771238 Free PMC article.
-
Mapping Excited-State Decay Mechanisms in Acetylacetone by Sub-20 fs Time-Resolved Photoelectron Spectroscopy.J Am Chem Soc. 2025 Aug 27;147(34):30785-30793. doi: 10.1021/jacs.5c06327. Epub 2025 Aug 13. J Am Chem Soc. 2025. PMID: 40801839 Free PMC article.
-
Excimer Intermediates en Route to Long-Lived Charge-Transfer States in Single-Stranded Adenine DNA as Revealed by Nonadiabatic Dynamics.J Phys Chem Lett. 2020 Sep 17;11(18):7483-7488. doi: 10.1021/acs.jpclett.0c02193. Epub 2020 Aug 26. J Phys Chem Lett. 2020. PMID: 32794719 Free PMC article.
References
-
- Beckstead A. A., Zhang Y., de Vries M. S., Kohler B. Phys. Chem. Chem. Phys. 2016;18:24228–24238. - PubMed
-
- Serrano-Andrés L., Merchán M. J. Photochem. Photobiol., C. 2009;10:21–32.
-
- Giussani A., Segarra-Martí J., Roca-Sanjuán D., Merchán M. Top. Curr. Chem. 2015;355:57–97. - PubMed
-
- Improta R., Santoro F., Blancafort L. Chem. Rev. 2016;116:3540–3593. - PubMed
-
- Crespo-Hernandez C. E., Cohen B., Hare P. M., Kohler B. Chem. Rev. 2004;104:1977–2019. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

