Effects of high intensity interval training on exercise capacity in people with cystic fibrosis: study protocol for a randomised controlled trial
- PMID: 30450213
- PMCID: PMC6219072
- DOI: 10.1186/s13102-018-0108-2
Effects of high intensity interval training on exercise capacity in people with cystic fibrosis: study protocol for a randomised controlled trial
Abstract
Background: In people with cystic fibrosis (CF), higher exercise capacity is associated with better health-related quality of life (HRQoL), reduced risk of hospitalisation for a respiratory infection and survival. Therefore, optimisation of exercise capacity is an important treatment goal. The Australian and New Zealand clinical practice guidelines recommend that people with CF complete 30 to 60 min of moderate intensity aerobic exercise on most days of the week. This recommendation can be difficult to achieve by people with CF because of time constraints, and intolerable breathlessness and muscle fatigue during continuous exercise. In contrast, a low-volume, high intensity interval training (HIIT) program may be a more achievable and efficient training method to improve exercise capacity in people with CF.
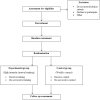
Methods: A randomised controlled trial will be undertaken. Forty people with CF (aged ≥15 years) will be randomly allocated, on a 1:1 ratio, to either the experimental or control group. Regardless of their group allocation, all participants will be asked to continue with their usual daily treatment for the study duration. Those in the experimental group will complete 8 weeks of thrice weekly HIIT on a cycle ergometer. Those in the control group will receive weekly contact with the investigators. The primary outcome of this study is exercise capacity. Secondary outcomes are HRQoL, exercise self-efficacy, feelings of anxiety, depression and enjoyment. These outcomes will be recorded at baseline (i.e. prior to randomisation) and following the 8-week intervention period. The study will also report other outcomes of the HIIT program (cardiovascular responses, symptom response, post-exercise muscle soreness and tolerance) and behaviour change techniques such as reinforcement, feedback and goal setting, used during the HIIT program.
Discussion: This study will determine the effects of 8-weeks of supervised, low-volume HIIT, completed on a cycle ergometer on measures of exercise capacity, HRQoL, exercise self-efficacy, feelings of anxiety, depression and enjoyment. If effective, this type of training could be an attractive alternative to traditional continuous training because it may be more achievable and time efficient.
Trial registration: Australian and New Zealand Clinical Trials Registry (ANZCTR):12617001271392 (04/09/2017).
Keywords: Cystic fibrosis; Exercise; High intensity interval training.
Conflict of interest statement
This protocol is approved by the Human Research Ethics Committee at Sir Charles Gairdner Hospital, on behalf of Sir Charles Gairdner Hospital and Perth Children’s Hospital (RGS 0000000065) with reciprocal approval from Curtin University (HRE2017–0651). Written informed consent will be obtained from each participant.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Orenstein D. Cystic fibrosis. Respir Care. 1991;36:746–754.
-
- Keogh RH, Szczesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: a longitudinal study using UK patient registry data. J Cyst Fibros. 2018;17(2):218–227. doi: 10.1016/j.jcf.2017.11.019. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources